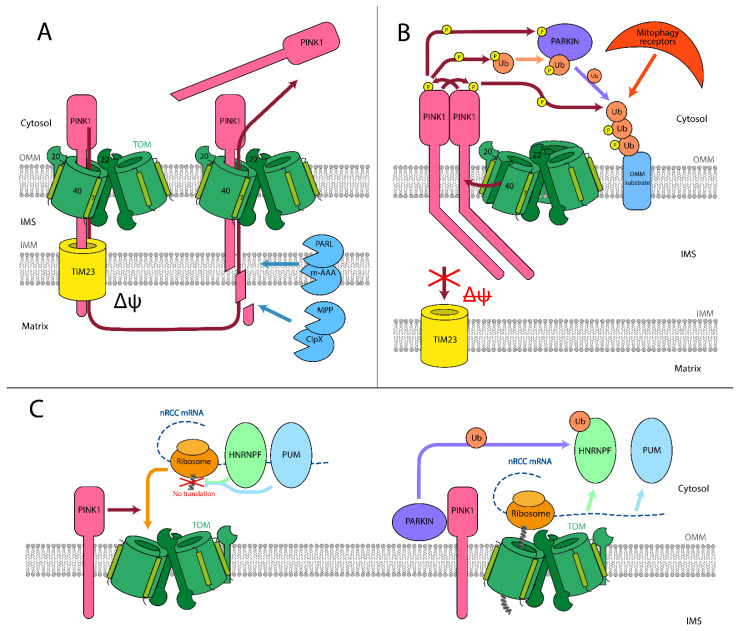
Figure 2.
Role of mitochondrial protein import in Parkinson’s disease. (A) PINK1/PARKIN mitochondrial quality control model. In healthy mitochondria, PINK1 is transported through both TOM and TIM complexes, utilizing the IMM electrostatic potential. Afterward, it is cleaved inside the matrix and on the IMM by mitochondrial proteases. The remaining part of PINK1 is dislocated into the cytosol, where it is degraded. This process allows keeping PINK1 levels on the OMM negligible. (B) In unhealthy mitochondria, PINK1 is unable to be transported through the TIM complex. It is ejected from TOMM40 laterally into the OMM, and the ejection is facilitated by TOMM7. PINK1 remains in connection with TOMM20. In that state, it accumulates on the OMM and starts forming homodimers within an HMW PINK1 complex. In dimeric form, PINK1 phosphorylates its dimeric partner and starts to phosphorylate PARKIN and ubiquitin. Phosphorylated PARKIN and ubiquitin form complex and ubiquitinate targets on the OMM. PINK1 phosphorylates ubiquitin on such targets, which attracts mitophagy receptors, starting the mitophagy process. (C) Proposed nRCC translation regulation mechanism. While the nRCC mRNA is in the cytosol and away from the mitochondria, PUM and HNRNPF are attached to it and serve as translation repressors. PINK1 attracts mRCC mRNA to the TOM complex on the outer mitochondrial membrane. Then, PARKIN removes the translation repressors HNRNPF and PUM from the mRNA. For HNRNPF protein, this process involves ubiquitination by PARKIN. When the repressors are released, co-translational synthesis through the TOM complex starts.