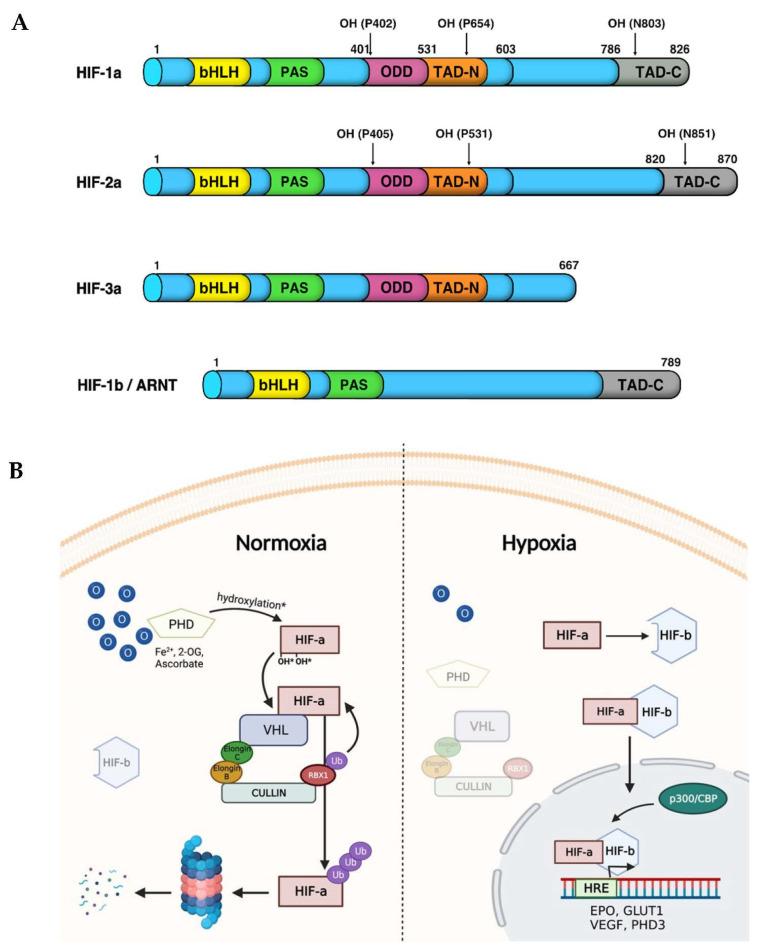
Figure 3.
HIF-α isoforms and their receptor HIF-1β/ARNT structural domains. (A) In vertebrates both HIF-1α and HIF-2α, but not HIF-3α, contain a basic helix-loop-helix (bHLH) domain, a Per-Arnt-Sim domain (PAS), an oxygen dependent degradation (ODD) domain, an N-terminal transactivation domain (N-TAD) located in the ODD and a C-TAD localized in the C-terminal region. (B) The HIF signalling pathway. Under normoxic conditions, PHDs hydroxylate HIF-α subunits on two specific prolyl residues within the ODDD. In turn, VHL recognizes and binds to hydroxylated HIF-α and then recruits the other components of the E3-ubiquitin ligase complex. The latter promotes the ubiquitin-mediated proteasomal degradation of HIF-α subunits. Conversely, hypoxic conditions inhibit PHD activity, and the subsequent degradation of HIF-α, which can, in turn, be stabilized in the cytoplasm, can dimerize with HIF-β-subunit and migrate into the nucleus. Here, the HIF-αβ active complex enhance the expression of target genes such as PHD3, VEGF, GLUT1 and EPO involved in restoring oxygen homeostasis. Created with BioRender.com.