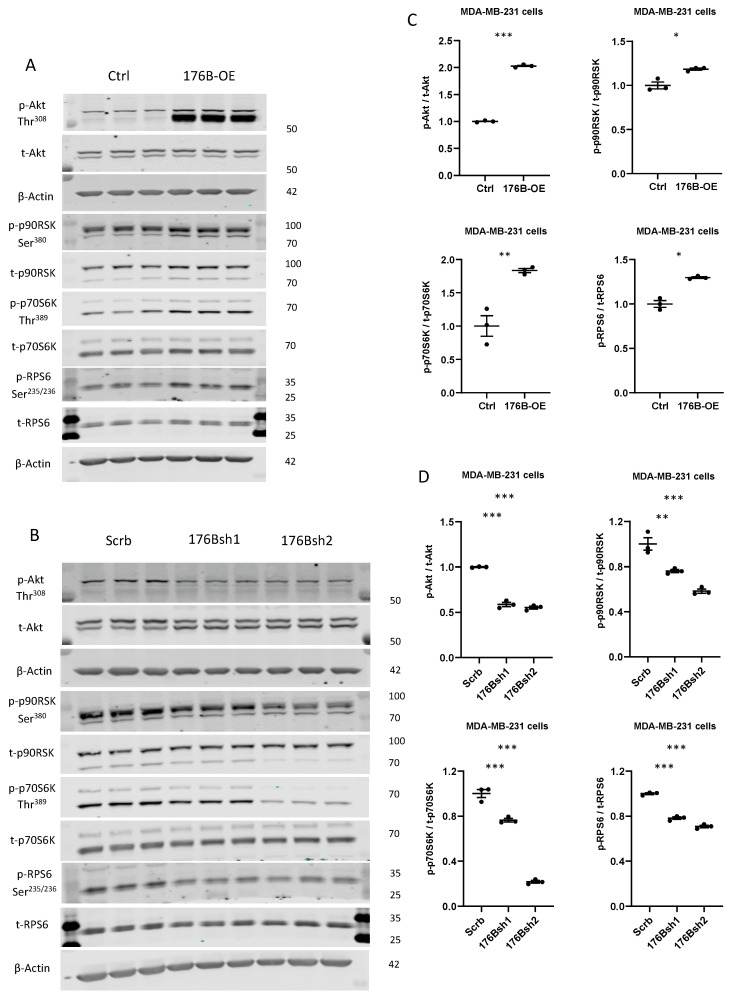
Figure 6.
TMEM176B overexpression increased AKT/mTOR pathway activation, which was decreased in TMEM176B-silenced cells. (A) Representative Western blot of protein lysates from control (Ctrl) and TMEM176B-overexpressing (176B-OE) cells and (B) shRNA control (Scrb) and TMEM176B-silenced (176Bsh1, 176Bsh2) cells examining phospho-AKT (Thr308) and total AKT, phospho-p90RSK (Ser380), total- p90RSK, phospho-p70S6K (Thr389), total-p70S6K, phospho-RPS6 (Ser235/236), and total-RPS6 and β-Actin. (C,D) Densitometry analysis of Western blot was performed using the ImageJ software (n = 3 per group, with three independent experiments). Data are presented as means ± SEM. Differences between groups were evaluated by Student’s t-test (C) and the one-way (D) ANOVA test with the Bonferroni post hoc test. * p < 0.05, ** p < 0.01, *** p < 0.001. 70-kDa ribosomal protein S6 kinase (p70S6K) and ribosomal protein S6 (RPS6) (A,C). Conversely, in the TMEM176B-silenced cells, we found reductions in AKT (Thr308) p90RSK, p70S6K, and RPS6 phosphorylation compared with control cells (B,D). These results suggest that the cation channel TMEM176B regulates the AKT/mTOR signaling pathway.