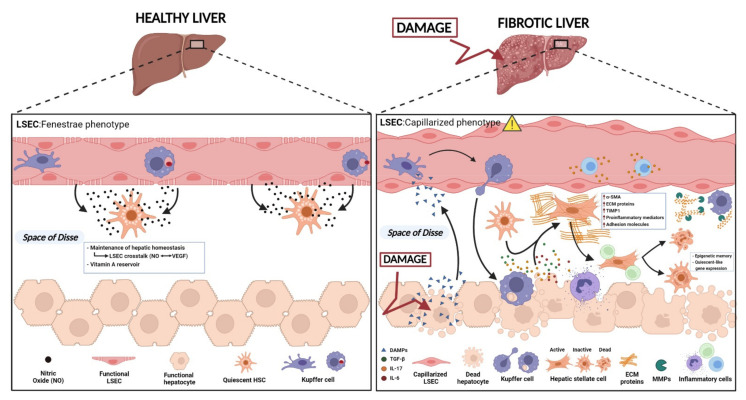
Figure 1.
Progression of hepatic fibrosis. Under physiological conditions, HSCs are found in a quiescent state where they function as pericytes and reservoirs of retinol (vitamin A). Retinol, together with other lipids, is stored in perinuclear cytoplasmic lipid droplets. Liver sinusoidal endothelial cells (LSECs) retain HSCs quiescent state by the releasing of nitric oxide (NO). When the liver is injured, HSCs transdifferentiate into a myofibroblast-like cell with a high proliferative and secretory phenotype. This occurs as a consequence of different factors. Firstly, LSECs change their phenotype into a capillarized structure while stopping the release of NO. Damaged hepatocytes release damage-associated molecular patterns (DAMPs) that attract and activate Kupffer cells and other inflammatory cells. These macrophages go to the injury site and release pro-inflammatory and pro-fibrogenic cytokines such as TGF-B, IL-17, and IL-6, inducing the activation of HSCs. In this activated state, HSCs acquire a high proliferative, secretory phenotype, where the perinuclear lipid droplets are lost and high levels of alpha smooth muscle actin (α-SMA) are transcribed in an attempt to help the cell migrate to the site of injury. Together with ECM molecules, the activated HSCs secrete molecules of tissue inhibitor of metalloproteinase-1 (TIMP1) to control matrix degradation. Cytokines and growth factors that help to repair injured liver tissue, as well as different pro-inflammatory mediators and adhesion molecules are secreted to recruit resident and circulating immune cells, thus further contributing to the perpetuation of fibrosis. The figure was created with BioRender.com.