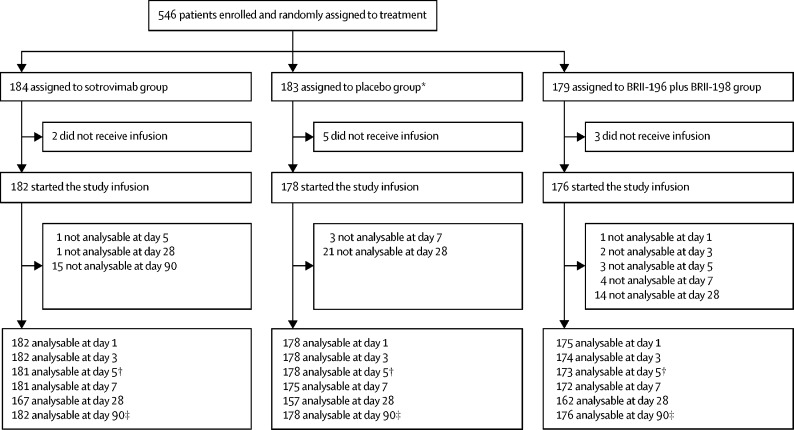
Figure 1.
Study profile
*One patient who was randomly assigned to the placebo group was enrolled at a site not using the BRII-196 plus BRII-198 agent. This patient was not included in the placebo group for comparisons with BRII-196 plus BRII-198. †Day 5 outcomes were used for the early futility assessment. ‡Day 90 outcome of sustained clinical recovery was the primary efficacy outcome; patients lost to follow-up were censored.