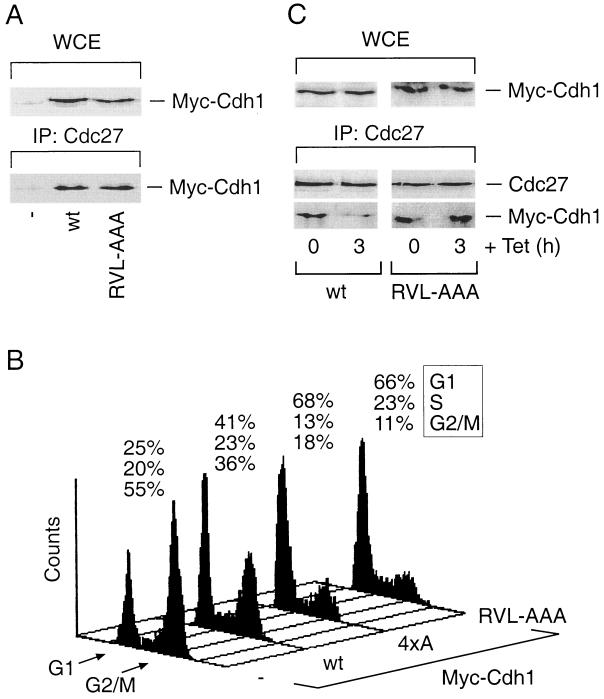
FIG. 4.
Disruption of the cyclin-binding motif of Cdh1 delays its dissociation from the APC and induces prolonged G1/S block. (A) U-2-OS cells were transfected as for Fig. 3B. After 36 h, cell lysates were prepared and analyzed either for the total levels of both wild-type (wt) and RVL-AAA Cdh1 proteins in whole-cell extracts (WCE) or for their presence within the Cdc27 immunocomplexes by Western blotting using the antimyc antibody. IP, immunoprecipitation. (B) U-2-OS cells were transiently transfected with the pXmyc expression plasmid containing either no insert (−) or the wild-type, cyclin binding-deficient (RVL-AAA) and phosphorylation-deficient (4xA) versions of Cdh1. In each case, the expression plasmid for the CD20 surface marker was also included. After 36 h, the culture medium was supplemented with nocodazole (40 ng/ml) for another 12 h in order to trap cells in mitosis. Productively transfected cells were sorted and analyzed for their DNA content by flow cytometry. Positions of the peaks corresponding to G1 and G2/M cells are indicated, as well as the percentages of cells in distinct cell cycle phases at the end of the assay period. (C) U-2-OS cells containing tetracycline (Tet)-regulated myc-tagged versions of the wild-type and the cyclin binding-deficient (RVL-AAA) forms of Cdh1 were induced to express the transgenes for 12 h by culturing in a Tet-free medium. When indicated, transcription of the transgenes was inhibited by readdition of Tet for another 3 h. The cell extracts were subsequently analyzed for total levels of the ectopically expressed Cdh1 proteins (WCE) and for their presence in the Cdc27 immunocomplexes by Western blotting with the antimyc antibody.