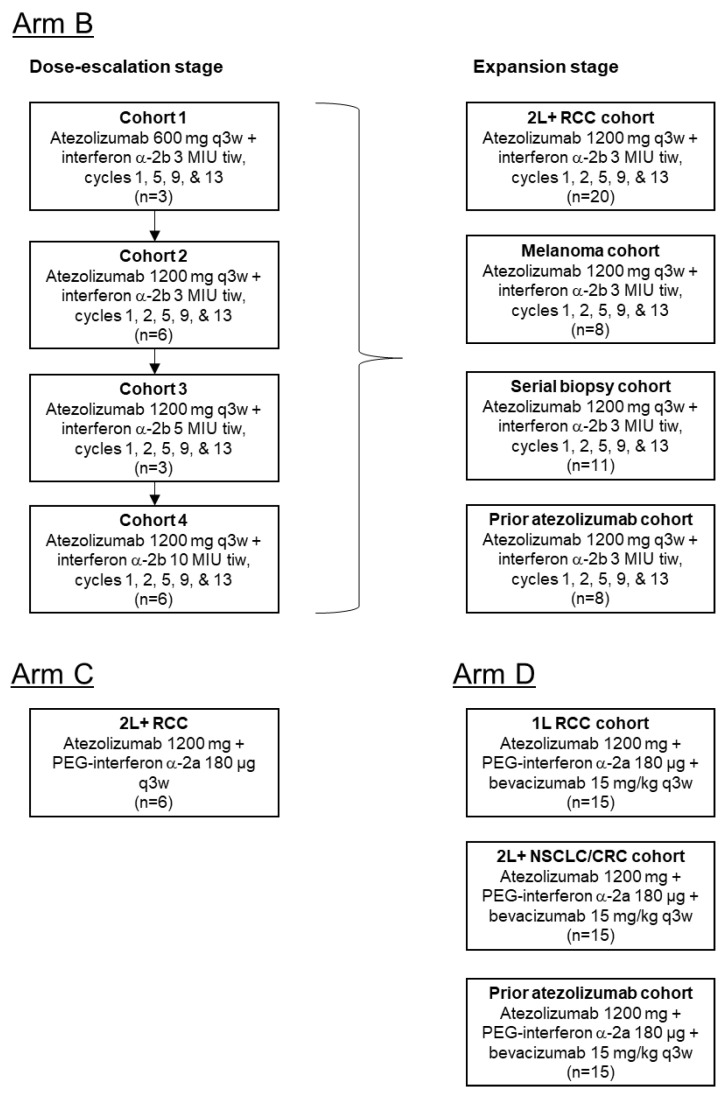
Figure 1.
Patient disposition. A total of 116 patients were enrolled into Arms B, C, and D of the study. Arm B recruited 65 patients with previously treated RCC or melanoma into 4 cohorts, with each cohort receiving different dosing regimens of atezolizumab + interferon-a-2b in the initial dose-escalation stage. At the dose expansion stage, patients in Arm B received atezolizumab 1200 mg q3w + interferon a-2b 3 MIU tiw. Arm C comprised 6 patients with previously treated RCC, and they received atezolizumab 1200 mg + PEG-interferon a-2a 180 µg q3w. Arm D recruited 45 patients into 3 cohorts, with each patient receiving atezolizumab 1200 mg + PEG-interferon a-2a 180 µg + bevacizumab 15 mg/kg q3w. MIU, million international units; q3w, every 3 weeks; tiw, 3 times per week.