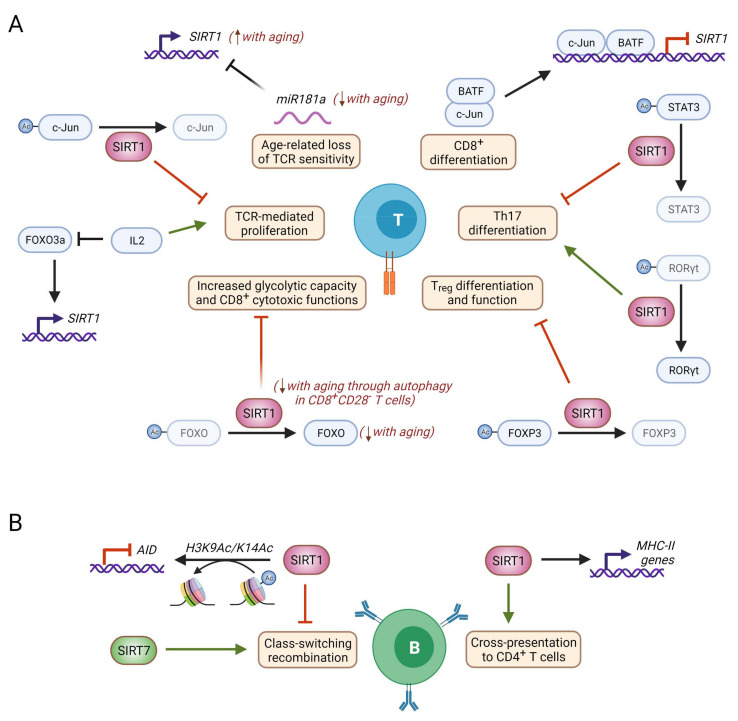
Figure 6.
Molecular mechanisms of nuclear sirtuin activity in B and T cells. (A) SIRT1 negatively regulates TCR-mediated T cell activation through deacetylation of the AP-1 member c-Jun [40,95,96,97]. Conversely, IL-2 induces exit from anergy by repressing SIRT1 expression via inhibition of FOXO3a, and BAFT cooperates with c-Jun to repress SIRT1 expression during CD8+ differentiation [98,99]. Age-associated miR181a downregulation in T cells results in SIRT1 upregulation, leading to a loss of TCR sensitivity [100]. SIRT1 balances Th17 differentiation through two opposite mechanisms: on the one hand, SIRT1 deacetylates STAT3, thereby impairing STAT3 nuclear translocation and transcription of the STAT3 target Rorc [41]. Contrarily, SIRT1-mediated deacetylation of RORγt is needed for its proper transcriptional activity. SIRT1 also negatively regulates Treg differentiation and suppressive functions through deacetylation of FOXP3, which enhances its proteasomal degradation [101]. In CD8+CD28- T cells, which accumulate during aging, SIRT1 protein levels are dramatically decreased, resulting in a reduction of FOXO1 acetylation and half-life that contributes to increased CD8+ cytotoxicity [102,103]. (B) In B cells, SIRT1 deficiency results in reduced levels of MHC-II, which results in impaired cross-presentation to CD4+ T cells [104]. SIRT1 is also important for class switching recombination, as it represses AID expression by deacetylation of H3K9Ac and H3K14Ac at the AID promoter [46]. Contrarily, Sirt7-/- splenic B cells display defective class switching recombination [18]. Faded lines indicate age-related loss of function, and comments in red indicate age-related changes. Figure created with BioRender.com.