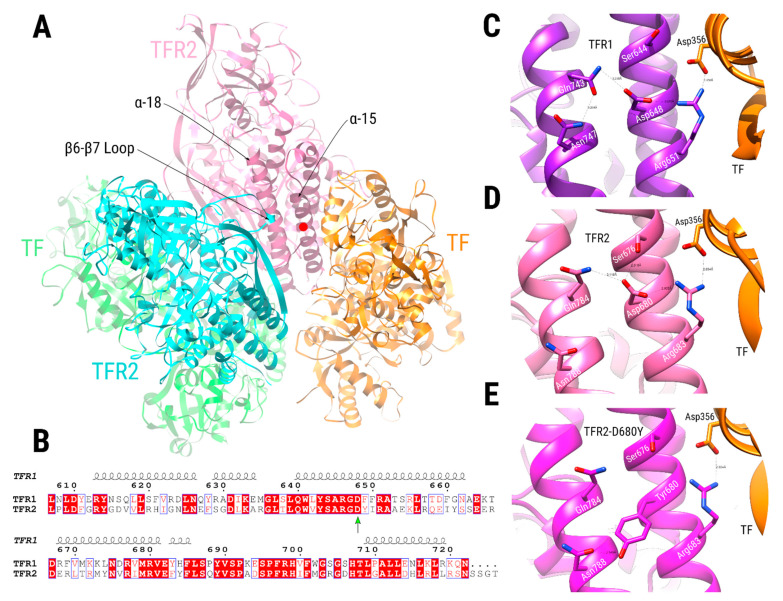
Figure 5.
Comparative modeling of the TFR2 dimer complexed with TF. (A) TFR2 chains are shown in cyan and pink, while TF chains are shown in green and orange. Dimeric interactions between TFR2 chains, close to Asp680 position (red circle at the α-15 helix, pink chain), are made between residues in the α-18 helix of one TFR2 chain (pink chain) and the loop located between the β-6 and β-7 strands of the other (cyan chain). (B) Sequence alignment of the TFR1 and TFR2 proteins near the TFR2 680 position (green arrow). Numbering is according to the TFR1 sequence, and the secondary structure depiction is based on the TFR1 structure (PDB ID 1CX8). The alignment coloring was produced by the ESPript 3.0 web server [40], where position with red background means absolute sequence conservation. (C–E) Structural context of positions TFR1-Asp648 (C, purple), TFR2-Asp680 (D, pink), and TFR2-Asp680Tyr (E, magenta). Residue names of TFR1/2 appear in white, while residues in TF are in black. Significant interactions are shown with dashed lines.