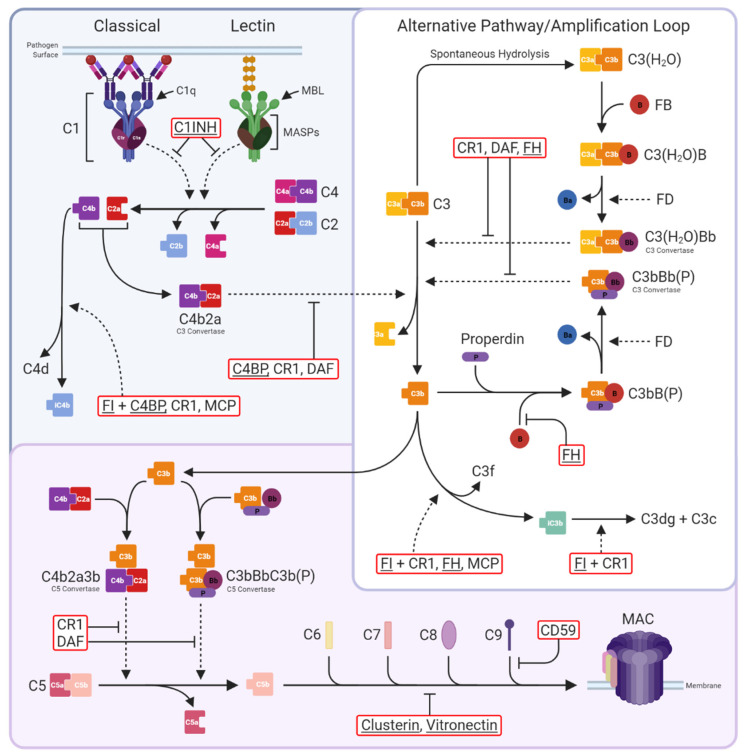
Figure 1.
The complement system. Three activation pathways converge on the central C3 molecule. The classical pathway is triggered by binding of antibody-antigen complexes to C1 via C1q subunits. C1r proteolytically activates C1s, which in turn cleaves C4 and C2 to form the classical C3 convertase C4b2a. The lectin pathway begins with recognition of pathogen surface carbohydrates by mannose-binding lectin (MBL) followed by activation of MBL-associated serine proteases (MASPs), which also cleave C4 and C2 to generate C4b2a. The alternative pathway is an amplification loop initiated by C3b generated in the above activation pathways or by spontaneous hydrolysis of C3 to C3(H2O). Factor B (FB) then binds C3b/C3(H2O), enabling its cleavage by Factor D (FD) to form the alternative pathway C3 convertase C3bBb/C3(H2O)Bb; binding of properdin (P) stabilises the convertase. Both C3 convertases cleave C3 into C3a and C3b. The classical and lectin pathways are negatively regulated by C1-inhibitor (C1INH), which inhibits both C1s and MASPs, while the C3 convertases are regulated by C4b-binding protein (C4BP; specific for C4b2a), decay-accelerating factor (DAF; specific for C3bBb), complement receptor 1 (CR1), and Factor H (FH), either directly through increasing decay or indirectly by catalysing cleavage of C4b by Factor I (FI). At the next stage of the pathway, C3b is incorporated into the C3 convertases to form the C5 convertases C4b2a3b and C3bBbC3b(P). These are regulated in the same manner as the C3 convertases and cleave C5 into C5a and C5b to trigger the terminal pathway. C5b is sequentially bound by C6, C7, C8, and up to 18 C9 molecules to form the membrane attack complex (MAC); MAC assembly is inhibited by clusterin and vitronectin in the fluid phase and CD59 on cells. Complement regulators are in red boxes, fluid-phase regulators are underlined. Solid, dotted, and blunt arrows indicate pathway progression, proteolytic cleavage, and direct inhibition, respectively.