Figure 1. Recommendation Sources Informing Clinical Pharmacogenetic Recommendations by Therapeutic Area.
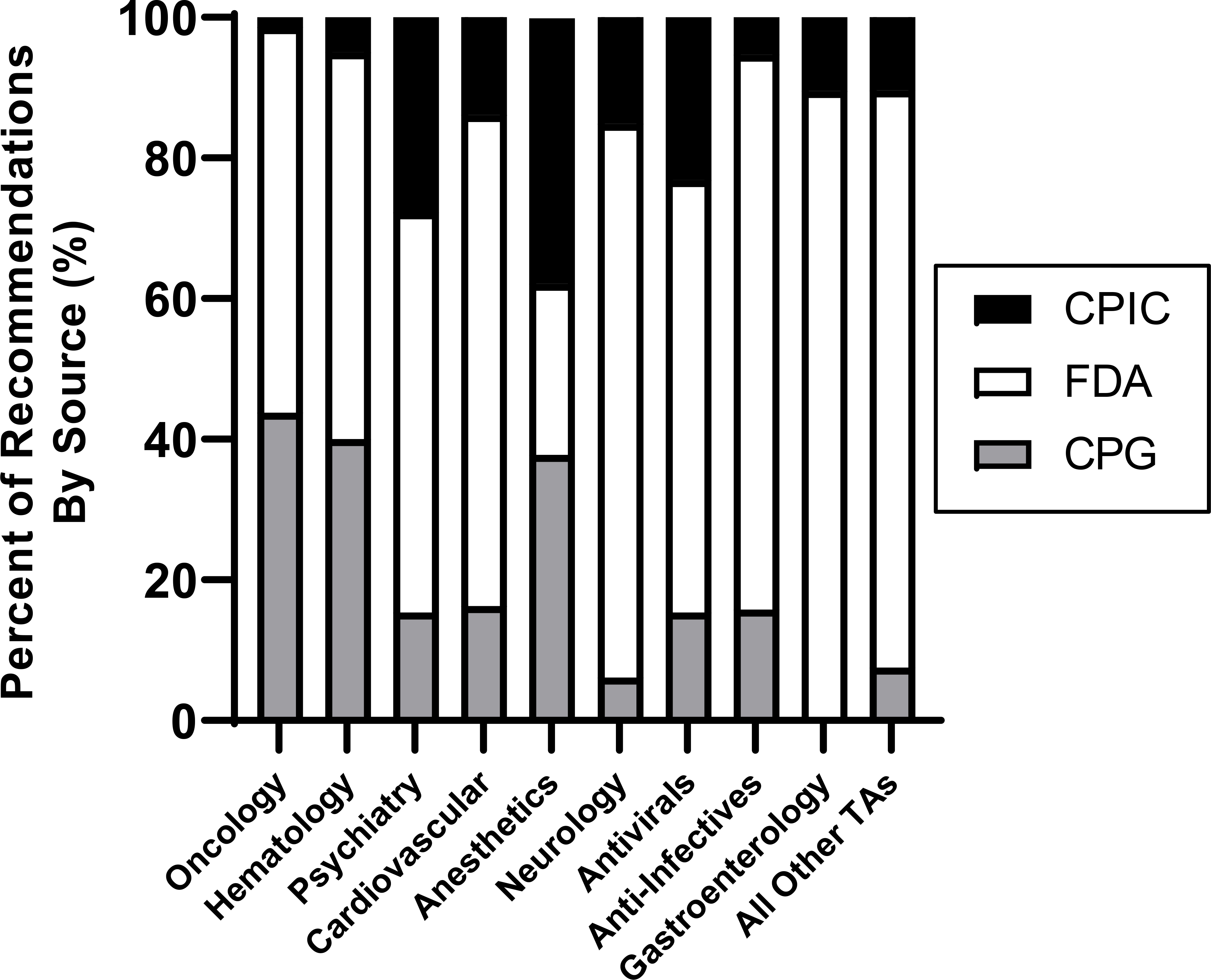
As collected from 302 pharmacogenetic recommendation documents, the total number of recommendations from each source (i.e., CPIC, FDA, or CPGs) are displayed as a percentage of the total number of recommendations for each therapeutic area. The “all other therapeutic areas” category includes pulmonary, rheumatology, endocrinology, analgesic, urology, immunosuppressant, medical countermeasure, metabolism, reproduction, addiction, and bone products.
Abbreviations: CPG = Clinical Practice Guidelines from U.S. Professional Organizations; CPIC = Clinical Pharmacogenetics Implementation Consortium; FDA = U.S. Food and Drug Administration; TA = therapeutic area
