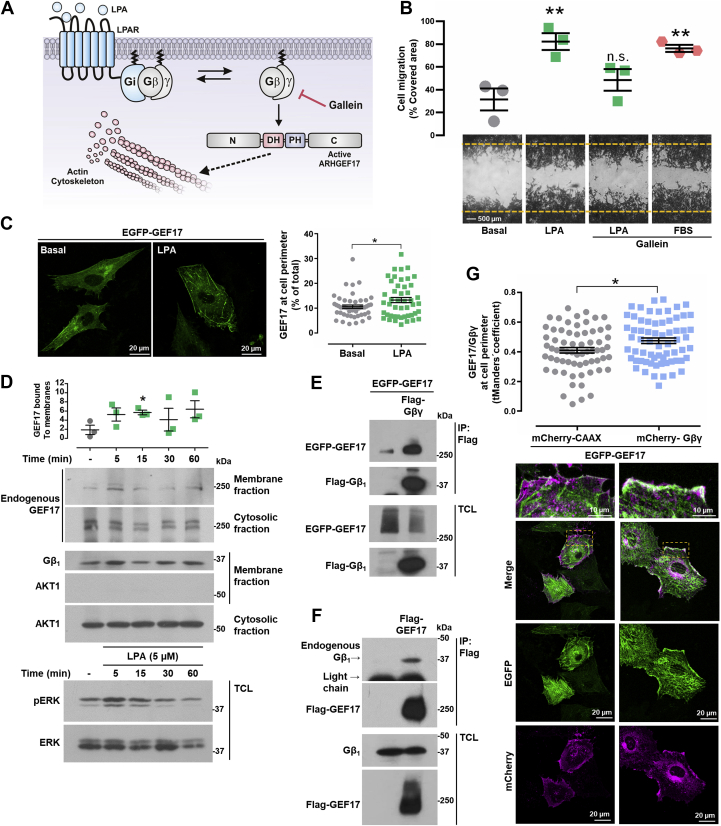
Figure 5.
Gβγ recruits ARHGEF17.A, hypothetical model representing the role of Gβγ (tested with gallein) on ARHGEF17 localization and activity. B, migration of LAP0297 cells preincubated or not with gallein (10 μM) was stimulated with LPA (5 μM), FBS served as control. Data represent the mean ± SEM, n = 3; ∗∗p < 0.01, one-way ANOVA followed Dunnett's test; ns. C, PAE cells were transfected with EGFP-ARHGEF17 and stimulated with LPA (5 μM) for 30 min, fixed and visualized by confocal microscopy. Graph represents the mean ± SEM of GEF17 detected at the cell perimeter (percent of total, 50, and 47 cells, in basal or stimulated conditions, respectively, was analyzed with FIJI-ImageJ software). ∗p < 0.05; t test. The scale represents 20 μm. D, LAP0297 cells were stimulated with LPA (5 μM) for the indicated times, and membrane and cytosolic fractions were obtained as described in the Experimental procedures section and analyzed by Western blot to detect the fraction of ARHGEF17 recruited to the membrane. Heterodimeric Gβγ, revealed by Western blot against Gβ1, served as membrane marker and for normalization of ARHGEF17 bound to the membrane fraction. AKT1 served as cytosolic marker. Cell stimulation was confirmed by detecting the phosphorylation of ERK (pERK). A representative blot from three independent experiments is shown. Graph represents the mean ± SEM. n = 3. ∗p < 0.05; t test. E, coimmunoprecipitation of GEF17 and Gβγ was analyzed using lysates from HEK 293T cells transfected with EGFP-GEF17 together or not with FLAG-Gβγ. Immunoprecipitation was done with FLAG antibodies. GEF17 and Gβ1 were detected by Western blot. A representative blot from three independent experiments is shown. F, FLAG-GEF17 was immunoprecipitated from transfected HEK 293T cells, and interacting endogenous Gβγ was detected by Western blot with antibodies against Gβ1. Western blot is representative of three independent experiments. G, effect of Gβγ on ARHGEF17 localization was analyzed by confocal fluorescence microscopy of PAE cells expressing EGFP-GEF17 with mCherry-Gβ1γ2 or mCherry-CAAX, as control. Graph represents the t Mander's coefficient of colocalization of EGFP-ARHGEF17 with mCherry-Gβγ (66 cells per condition, from three independent experiments, were analyzed with the Coloc2 plugin of the FIJI-ImageJ software). Graph represents the mean ± SEM. ∗p < 0.05; t test. Representative cells are shown below the graph. The scale represents 20 μm; zoom represents 10 μm. EGFP, enhanced GFP; ERK, extracellular-regulated kinase; FBS, fetal bovine serum; HEK 293T, human embryonic kidney 293T cell line; LPA, lysophosphatidic acid; ns, nonsignificant; PAE, porcine aortic endothelial.