Figure 9.
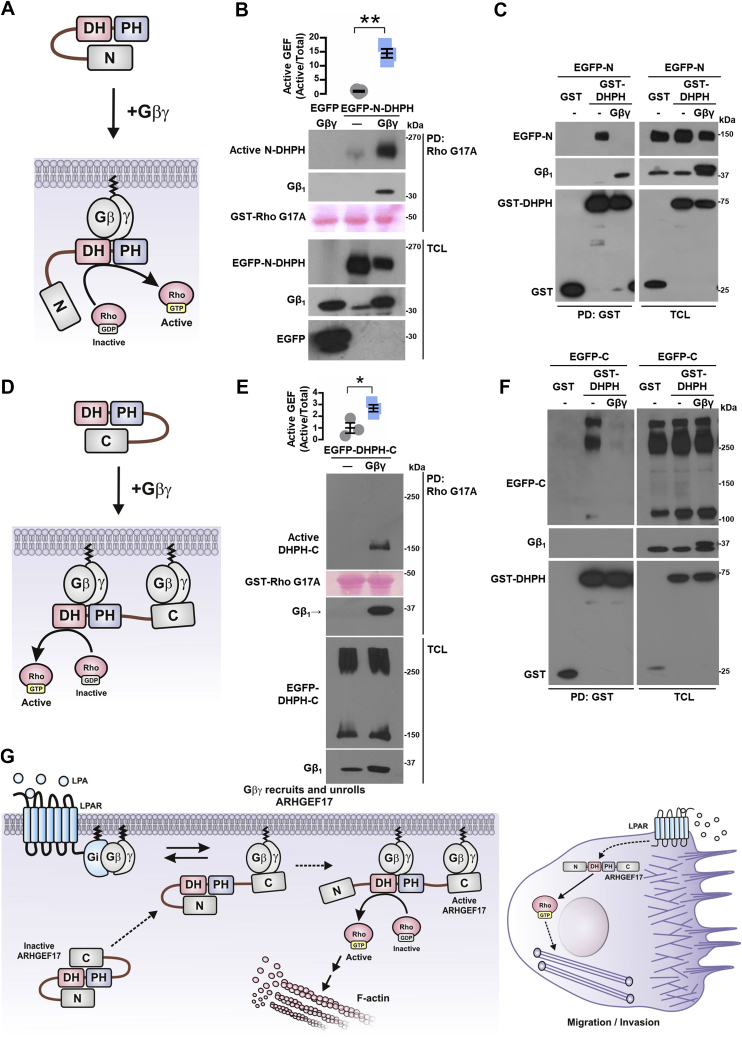
Gβγ activates ARHGEF17 by displacing intramolecular interactions.A, hypothetical model representing how Gβγ activates the GEF17-N-DHPH construct. B, active GEF17-N-DHPH was isolated from HEK 293T cells cotransfected or not with Gβγ. Graph represents the mean ± SEM from three independent experiments. ∗∗p < 0.01; t test. C, GST-GEF17-DHPH or GST (as control) was isolated by pull down from HEK 293T cotransfected with GFP-GEF17-N domain (EGFP-N) in the presence or the absence of Gβγ. Transfected proteins were detected by Western blot in pull downs and total cell lysates. Blot is representative of four independent experiments. D, hypothetical model representing how Gβγ activates the GEF17-DHPH-C construct. E, active GEF17-DHPH-C was isolated from HEK 293T cells cotransfected with EGFP-GEF17-DHPH-C construct in the presence or the absence of Gβγ. Graph represents the mean ± SEM from three independent experiments. ∗p < 0.05; t test. F, GST-GEF17-DHPH or GST (as control) was isolated by pull down from HEK 293T cells cotransfected with EGFP-GEF17-C domain (EGFP-C) in the presence or the absence of Gβγ. Transfected proteins were detected by Western blot in pull downs and total cell lysates. Blot is representative of four independent experiments. G, model depicting how ARHGEF17 is recruited and activated by Gβγ. Gi-coupled receptors release Gβγ from Gi. Gβγ recruits ARHGEF17 to the plasma membrane by interaction with the C-terminal domain of the RhoGEF, disrupting an inhibitory interaction that hides the DH–PH domains. A second Gβγ heterodimer interacts with GEF17-DH-PH domains promoting dissociation of GEF17 N-terminal domain, unleashing RhoGEF activity and exposing an actin-binding site at the N-terminal domain contributing to localize GEF17 to the actin cytoskeleton. These dynamic interactions coordinate the spatiotemporal activation of GEF17 during cell migration and invasion. EGFP, enhanced GFP; GST, glutathione-S-transferase; HEK 293T, human embryonic kidney 293T cell line; RhoGEF, Rho guanine nucleotide exchange factor.