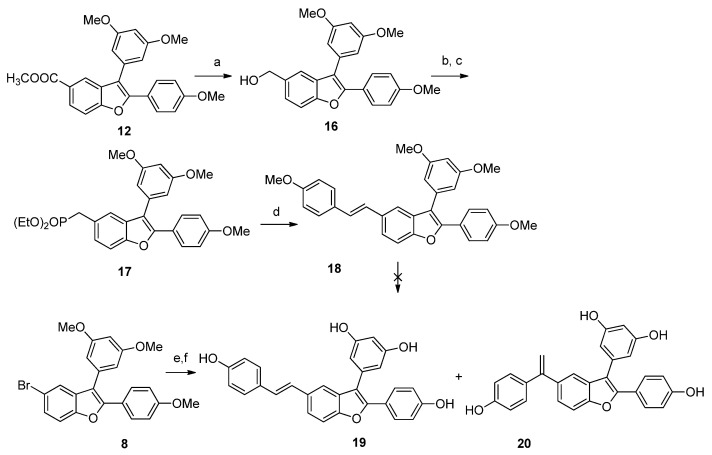
Scheme 3.
Reagents and conditions: (a) LiAlH4, THF, 0 °C, 20 min, quantitative yield; (b) PBr3, cat pyridine, Et2O, rt to reflux, 2 h, (c) P(OEt)3, 130 °C, overnight, 80% over two steps; (d) 4-methoxybenzaldehyde, NaH, 120 °C, 30 min, MW, 86%; (e) BBr3 1 M DCM, DCM, −78 °C to rt, overnight, 87%; (f) 4-hydroxystirene, TEA, dppp (1,3-bis(diphenylphosphino)propane), Pd(OAc)2, dry DMF, 120 °C, 48 h.