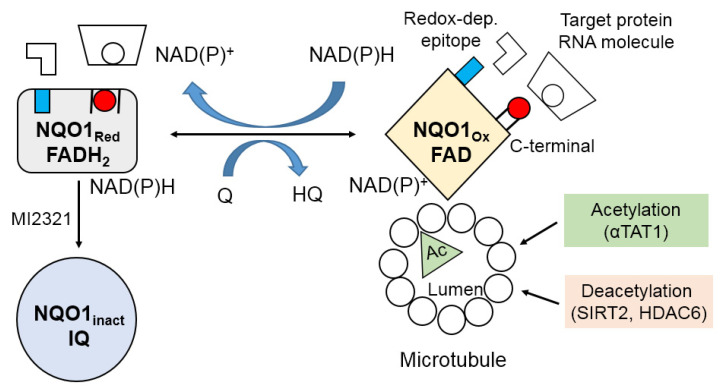
Figure 3.
Schematic representation of NQO1 as a molecular redox switch via conformational changes. The conformational changes of NQO1 can be attributed to the levels of reduced pyridine nucleotides. Under physiological conditions, adequate levels of NAD(P)H maintain NQO1 in the reduced form (FADH2), preventing antibodies from binding to the C-terminal domain and redox-dependent epitope. When NAD(P)H levels decrease, however, NQO1 takes on an oxidized form (FAD), exposing its C-terminal tail and redox-dependent epitope. The conformational change in NQO1 in response to the intracellular NAD(P)+/NAD(P)H redox balance modifies the binding of either RNA molecules or target proteins to NQO1. NAD(P)H levels also induce NQO1 conformational changes during binding to microtubules. NQO1 inactivation by the indolequinone component of inhibitor MI2321 modifies the conformation of NQO1, blocking its binding to microtubules. Decreased levels of NQO1 bound to microtubules result in decreased deacetylation or increased acetylation of lysine40 in α-tubulin in the microtubular lumen. Abbreviations—Red: reduced; Ox: oxidized; inact.: inactivated; Q: quinone; HO: hydroquinone; IQ: indolequinone; Ac: acetylated; αTAT1: alpha-tubulin N-acetyltransferase; SIRT2: NAD-dependent deacetylase sirtuin 2; HDAC6: histone deacetylase 6.