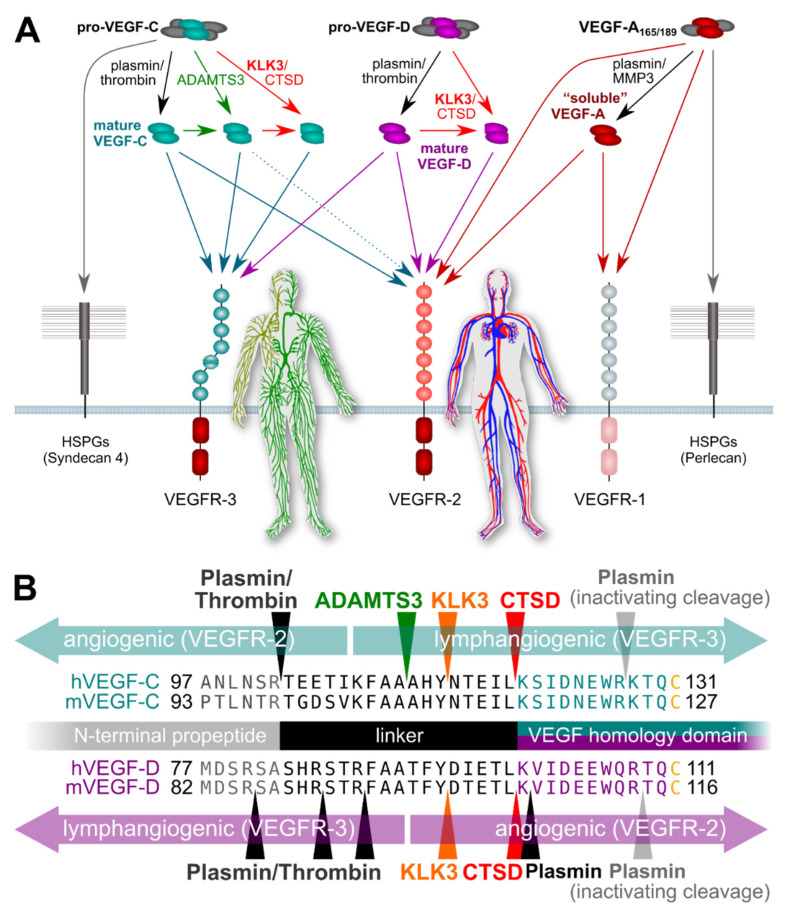
Figure 1.
Simplified schematic of the proteolytic processing of VEGFs. (A) In vitro data on the properties of different forms of mature VEGF-C and VEGF-D predict that KLK3 promotes lymphangiogenesis in tumors overexpressing VEGF-C, but angiogenesis in tumors overexpressing VEGF-D. Lymphangiogenesis is mediated via VEGFR-3, and progressive proteolytic processing renders VEGF-C selective for VEGFR-3. Unlike VEGF-C, VEGF-D can become exclusively angiogenic upon proteolytic processing [75]. VEGF-C- and VEGF-D mediated lymphangiogenesis could enhance tumor immune surveillance during the early stages of tumor development but promote metastasis (VEGF-C) or tumor angiogenesis (VEGF-D) during later stages. In the absence of proteolytic processing, the longer isoforms of VEGF-A, pro-VEGF-D and pro-VEGF-C are sequestered on cell surface heparan sulfate proteolglycans (HSPGs) and in the extracellular matrix. (B) The locations of proteolytic processing for proteases known to cleave VEGF-C and/or VEGF-D. Note that the processing sites for plasmin and thrombin for VEGF-D have not been experimentally determined and are only predicted based on the known cleavage specificities.