Table 3.
Pharmacogenomics of antithrombotic drugs.
| Antithrombotic Drugs | ||
|---|---|---|
| Vitamin K Antagonists | ||
| Drug | Properties | Pharmacogenetics |
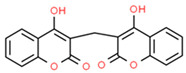
|
Name: Dicoumarol IUPAC Name: 4-hydroxy-3-[(4-hydroxy-2-oxo-2H-chromen-3-yl)methyl]-2H-chromen-2-one. Molecular Formula: C19H12O6 Molecular Weight: 336.295 Da. Mechanism: Inhibits vitamin K reductase, depletes vitamin KH2, cofactor for vitamin K-dependent protein-carboxylation, limits gamma-carboxylation, and activates vitamin K-dependent coagulant proteins. Inhibits synthesis of vitamin K-dependent coagulation factors II, VII, IX, and X and anticoagulant proteins C and S. Depresses vitamin K-dependent coagulation factors II, VII, and X, lowers prothrombin levels and the amount of fibrin-bound thrombin, reducing thrombogenicity. Effect: Antithrombotic agents. Vitamin K antagonists. |
Mechanistic genes:CRYZ, F2, F7, F9, F10, NQO1, PROC, PROS1, VKORC1 Metabolic genes Substrate:CYP2C9 Inhibitor:CYP2C6, CYP2C11 Transporter genes:ALB |
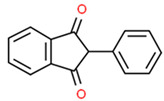
|
Name: Phenindione IUPAC Name: 2-phenyl-2,3-dihydro-1H-indene-1,3-dione. Molecular Formula: C15H10O2 Molecular Weight: 222.24 Da. Mechanism: Similar mode of action as Dicoumarol. Effect: Antithrombotic agents. Vitamin K antagonists. |
Mechanistic genes:ANXA5, F2, F7, F9, F10, PROC, PROS1, VKORC1 |
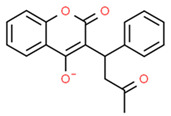
|
Name:Warfarin IUPAC Name, (1) 2H-1-benzopyran-2-one, 4-hydroxy-3-(3-oxo-1-phenylbutyl)-, sodium salt, (2) 3-(α-acetonylbenzyl)-4-hydroxycoumarin sodium salt Molecular Formula: C19H15NaO4 Molecular Weight: 330.31 Da Mechanism: Competitively inhibits subunit-1 of multi-unit VKOR complex, depleting vitamin K reserves. Antithrombogenic effects occur after functional coagulation factors IX and X are diminished. Phytonadione (vitamin K1) reverses anticoagulant effect. Slightly affects platelet-rich arterial thrombi-adherence to abnormal vessel wall. Effect: Antithrombotic agents, Anticoagulants, Coumarin Derivatives. Vitamin K Antagonist |
Mechanistic genes: F2, F5, F7, F9, F10, NR1I2, PROC, VKORC1 Metabolic genes Substrate: CALU, CYP1A2, CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP3A4, CYP3A5, EPHX1, GGCX Inhibitor: CYP2C9, CYP2C19, VKORC1 Inducer: CYP2C9, CYP3A4 Transporter genes: ABCB1, ALB, ORM1 Pleiotropic genes: APOE |
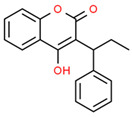
|
Name: Phenprocoumon IUPAC Name: 4-hydroxy-3-(1-phenylpropyl)-2H-chromen-2-one. Molecular Formula: C18H16O3 Molecular Weight: 280.32 Da. Mechanism: as per Dicoumarol Effect: Antithrombotic agents. Vitamin K antagonists. |
Mechanistic genes:F2, F7, F9, F10, PROC, PROS1, VKORC1 Metabolic genes Substrate:CYP2C8, CYP2C9, CYP3A4 Transporter genes:ALB, ORM1 |
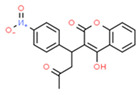
|
Name:Acenocoumarol IUPAC Name, (1) 2H-1-benzopyran-2-one, 4-hydroxy-3-[1-(4-nitrophenyl)-3-oxobutyl]-, (2) 3-(α-acetonyl-p-nitrobenzyl)-4-hydroxycoumarin Molecular Formula: C19H15NO6 Molecular Weight: 353.33 Da Mechanism: Interferes with hepatic synthesis of vitamin K-dependent coagulation factors II, VII, IX, X. Effect: Antithrombotic agents. Vitamin K antagonists. |
Mechanistic genes:CALU, F2, F7, F9, F10, VKORC1 Metabolic genes Substrate:CYP1A2, CYP2C9, CYP2C18, CYP2C19, CYP3A4 Transporter genes:ABCB1, ALB, ORM Pleiotropic gens: APOE |
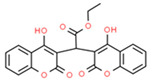
|
Name: Ethyl biscoumacetate IUPAC Name: ethyl 2,2-bis(4-hydroxy-2-oxochromen-3-yl)acetate. Molecular Formula: C22H16O8 Molecular Weight: 408.4 Da. Mechanism: Anticoagulant, mode of action similar to that of warfarin. Effect: Antithrombotic agents. Vitamin K antagonists. |
Mechanistic genes:F2, F7, F9, F10, VKORC1 Metabolic genes Substrate:CYP3A4 Inhibitor:GLUL |
| Heparins | ||
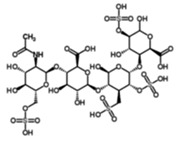
|
Name: Heparin IUPAC Name: (2S,3S,4R,5R,6R)-3-({(2R,3R,4R,5S,6R)-3-(acetylamino)-4,5-dihydroxy-6-[(sulfooxy)methyl]tetrahydro-2H-pyran-2-yl}oxy)-6-{[(2S,3S,4S,5R,6S)-6-{[(2R,3S,4S,5R)-2-carboxy-4,6-dihydroxy-5-(sulfooxy)tetrahydro-2H-pyran-3-yl]oxy}-2-hydroxy-4-(sulfomethyl)-5-(sulfooxy)tetrahydro-2H-pyran-3-yl]oxy}-4,5-dihydroxytetrahydro-2H-pyran-2-carboxylic acid. Molecular Formula: C26H41NO34S4. Molecular Weight: 1039.85 Da Mechanism: Potentiates antithrombin III activity; inactivates thrombin, coagulation factors IX, X, XI, XII, and plasmin; prevents conversion of fibrinogen to fibrin. Effect: Antithrombotic agents. Heparin group. |
Mechanistic genes:F9, F10, F11, F12, FCGR2A, FCGR3A, FGF1, FGF19, FGF2, FGF4, FGFR1, FGFR2, FGFR4, HGF, ITGB3, LIPA, PF4, PROC, SELP, SERPINA5, SERPINC1, VWF Metabolic genes Substrate:HPSE Transporter genes:ABCC1, SERPINA7 Pleiotropic genes:APP |
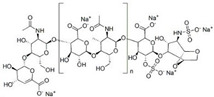
|
Name: Enoxaparin Mechanism: Enhances the inhibition rate of clotting proteases by antithrombin III, impairing normal hemostasis; strongly inhibits factor Xa. Effect: Antithrombotic agents. Heparin group. |
Mechanistic genes:ACE, F2, F5, F10, FCGR3A, IL1RN, ITGB3, MPO, SERPINC1, THBD Metabolic genes Substrate:HPSE Transporter genes:SERPINA7 |
| Platelet aggregation inhibitors | ||
|
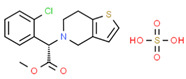
Name:Clopidogrel IUPAC Name: (1) Thieno[3,2-c]pyridine-5(4H)-acetic acid, α-(2-chlorophenyl)-6,7-dihydro-, methyl ester, (S)-, sulfate (1:1), (2) Methyl (+)-(S)-α-(o-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetate, sulfate (1:1) Molecular Formula: C16H16ClNO2S Molecular Weight: 419.90 Da Mechanism: Platelet inhibitor; irreversibly binds to P2Y12 ADP receptors on platelets preventing ADP binding to same receptors, activating the glycoprotein GPIIb/IIIa complex, and reducing platelet aggregation. Inhibits ADP-mediated release of platelet dense granule (e.g., ADP, Ca2+, and serotonin) and α-granule (e.g., fibrinogen and thrombospondin) contents that augment platelet aggregation. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:ITGA2B, ITGB3, P2RY12 Metabolic genes Substrate:CES1, CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5 Inhibitor:CYP2B6, CYP2C8, CYP2C9, CYP2C19 Transporter genes:ABCB1, SLC22A1, SLC22A2 |
|
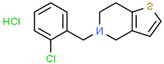
Name:Ticlopidine IUPAC Name, (1) Thieno[3,2-c]pyridine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydro-, hydrochloride, (2) 5-(o-chlorobenzyl)-4,5,6,7-tetrahydrothieno-[3,2-c]pyridine hydrochloride Molecular Formula: C14H14ClNS Molecular Weight: 300.25 Da Mechanism: Irreversibly blocks P2Y12 receptors as an active metabolite, preventing GPIIb/IIIa receptor complex activation, reducing platelet aggregation. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:ITGB3, P2RY12 Metabolic genes Substrate:CYP2B6, CYP2C19, CYP2D6, CYP3A4, MPO Inhibitor:CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 Pleiotropic genes:HLA-B |
|
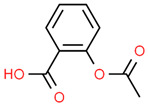
Name:Acetylsalicylic acid IUPAC Name: Benzoic acid, 2-(acetyloxy)-, (2) salicylic acid acetate Molecular Formula: C9H8O4 Molecular Weight: 180.16 Da Mechanism: Inhibits prostaglandin synthesis; acts on the preoptic area of the anterior hypothalamus to reduce fever. Blocks prostaglandin synthetase activity, preventing thromboxane A2 formation. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:AKR1C1, CASP1, CASP3, CCNA2, CCND1, EDNRA, GP1BA, GP6, HSPA5, IKBKB, MAP2K4; MYC, NFKBIA, PCNA, PRKAs, PTGER1, PTGER2, PTGER3, PTGER4, PTGES, PTGIR, PTGS1, PTGS2, RPS6KA3, TBX21, TBXA2R, TNFAIP6, TP53 Metabolic genes Substrate:ACSM1, CYP2C9, CYP3A4, GLYAT, NAT2, UGT1A1, UGT1A10, UGT1A3, UGT1A6, UGT1A7, UGT1A9, UGT2B4, UGT2B7 Inhibitor:CYP19A1, PTGS1, PTGS2 Inducer:CYP2C19, CYP2E1 Transporter genes, ABCB1, SLC22A6, SLC22A8 |
|
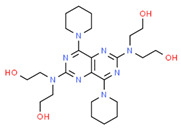
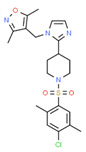
Name:Dipyridamole IUPAC Name: Ethanol, 2,2′,2′’,2′’’-[(4,8-di-1-piperidinylpyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetrakis-, (2) 2,2′,2′’,2′’-[(4,8-sipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]tetraethanol Molecular Formula: C24H40N8O4 Molecular Weight: 504.63 Da Mechanism: Non-nitrate coronary vasodilator; inhibits adenosine deaminase and phosphodiesterase activity, inducing accumulation of adenosine, adenine nucleotides, and cAMP which inhibit platelet aggregation, causing vasodilation. May stimulate prostacyclin or PGD2 activity. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:ADA, ORM1, PDE10A, PDE4A, PDE5A, PTGDR2, RCAN1 Transporter genes:ABCB1, ABCB11, ABCC4, ABCC5, SLCO1B1, SLCO1B3, SLCO2B1 |
|
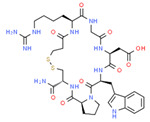
Name:Abciximab IUPAC Name: (1) Immunoglobulin G1, anti-(human integrin αIIbβ3) Fab fragment (human-mouse monoclonal c7E3 clone p7E3VHhCγ1 γ1-chain), disulfide with human-mouse monoclonal c7E3 clone p7E3VκhCκ κ-chain. Molecular Formula: C22H27ClN4O3S Molecular Weight: 462.993 Da Mechanism: Monoclonal antiglycoprotein IIb/IIIa receptor antibody; GPIIb/IIIa is the major platelet surface receptor in platelet aggregation. Blocks vitronectin receptor-mediated cell adhesion, and Mac-1 receptor on monocytes and neutrophils, inhibiting adhesion to monocyte cells. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. Glycoprotein IIb/IIIa Inhibitor. |
Mechanistic genes:FCGR2A, FCGR2B, ITGA2B, ITGB3, P2RY1, VTN Metabolic genes Substrate:CYP1A2, CYP2C19 |
|
Name:Eptifibatide IUPAC Name: (1) N6-amidino-N2-(3-mercaptopropionyl)-L-lysylglycyl-L-α-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide, cyclic (1–6)-disulfide. Molecular Formula: C35H49N11O9S2 Molecular Weight: 831.96 Da Mechanism: Blocks the platelet GPIIb/IIIa receptor, reversibly inhibiting platelet aggregation, preventing thrombosis. Effect: Antithrombotic agents, platelet aggregation inhibitors, and glycoprotein IIb/IIIa Inhibitors. |
Mechanistic genes:IL6, ITGA2B, ITGB3, P2RY1, TBXAS1 Metabolic genes Substrate:CYP1A2, CYP2C19 |
|
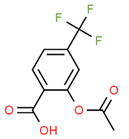
Name: Triflusal IUPAC Name: 2-(acetyloxy)-4-(trifluoromethyl)benzoic acid. Molecular Formula: C10H7F3O4 Molecular Weight: 248.15 Da Mechanism: irreversible COX-1 inhibitor in platelets; spares the arachidonic acid pathway in endothelial cells; favors nitric oxide production. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:NFKB1, NOS2, PDE10A, PTGS1 Metabolic genes Substrate:CYP2C8 Transporter genes:ALB |
|
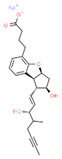
Name: Beraprost IUPAC Name: sodium 4-[(2S,3R,4R,6S)-4-hydroxy-3-[(1E,3S)-3-hydroxy-4-methyloct-1-en-6-yn-1-yl]-7-oxatricyclo[6.4.0.0^{2,6}]dodeca-1(8),9,11-trien-9-yl]butanoate. Molecular Formula: C24H29NaO5 Molecular Weight: 420.47 Da Mechanism: Binds prostacyclin membrane receptors, inhibiting Ca2+ release from intracellular stores, promoting vasodilation. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:
PTGIR
Metabolic genes Substrate: CYP2C8 |
|
Name: Treprostinil IUPAC Name: 2-{[(1R,2R,3aS,9aS)-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-1H,2H,3H,3aH,4H,9H,9aH-cyclopenta[b]naphthalen-5-yl]oxy}acetic acid. Molecular Formula: C23H34O5 Molecular Weight: 390.51 Da Mechanism: Prostacyclin vasodilator; binds to the prostacyclin receptor inducing vasodilation of pulmonary and systemic arterial vascular beds, inhibits platelet aggregation and inflammatory cytokine production. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:P2RY12, PPARD, PTGIR Metabolic genes Substrate:CYP2C9 |
|
Name,Prasugrel IUPAC Name: (1) 5-[(1RS)-2-cyclopropyl-1-(2-fluorophenyl)-2-oxoethyl]-4,5,6,7-tetrahydrothieno[3,2-c]pyridin-2-yl acetate hydrochloride. (2) 2-[2-(Acetyloxy)-6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl]-1-cyclopropyl-2-(2-fluorophenyl)ethanone hydrochloride Molecular Formula: C20H21ClFNO3S Molecular Weight: 409.90 Da Mechanism: P2Y12 platelet inhibitor; impairs ADP-mediated activation of the GPIIb/IIIa complex. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:P2RY12 Metabolic genes Substrate:CES1, CES2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP3A4, GSTs, POR Inhibitor:CYP2B6, CYP2C9, CYP2C19, CYP2D6, CYP3A4, CYP3A5 Transporter genes:ABCB1, ALB |
|
Name:Cilostazol IUPAC Name: (1) 2(1H)-quinolinone, 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-, (2) 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrocarbostyril Molecular Formula: C20H27N5O2 Molecular Weight: 369.46 Da Mechanism: Antiplatelet agent and vasodilator; inhibits PDE3 activation, increasing cAMP concentrations in platelets and blood vessels and mediating arterial vasodilation and inhibition of platelet aggregation. Reduces plasma triglyceride, but increases high-density lipoprotein cholesterol, levels. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. Phosphodiesterase Enzyme Inhibitor. |
Mechanistic genes:PDE3A Metabolic genes Substrate:CYP1A2, CYP1B1, CYP2C8, CYP2C19, CYP2D6, CYP3A4, CYP3A5, CYP3A7 Transporter genes:ABCB1 |
|
Name:Ticagrelor IUPAC Name: (1S,2S,3R,5S)-3-[7-[[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-propylsulfanyltriazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol Molecular Formula: C23H28F2N6O4S Molecular Weight: 522.57 Da Mechanism: P2Y12 platelet inhibitor; couples with Gαi2 and other Gi proteins to inhibit adenylyl cyclase. Activates PI3K, Akt, Rap1b, and K+ channels, mediating hemostasis and platelet aggregation. P2Y12 receptor blockade reduces development of occlusive thromboses, risk of MI and ischemic stroke. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. Selective adenosine diphosphate (ADP) receptor antagonist. |
Mechanistic genes:P2RY12 Metabolic genes Substrate:CYP2C19, CYP3A4, CYP3A5, UGTs Inhibitor:ABCB1, CYP1A2, CYP2C9, CYP3A4 Inducer:CYP2B6, CYP2C9 Transporter genes:ABCB1, ALB |
|
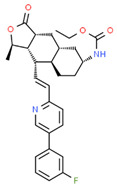
Name: Vorapaxar IUPAC Name: ethyl N-[(1R,3aR,4aR,6R,8aR,9S,9aS)-9-[(1E)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethenyl]-1-methyl-3-oxo-dodecahydronaphtho[2,3-c]furan-6-yl]carbamate. Molecular Formula: C29H33FN2O4 Molecular Weight: 492.58 Da Mechanism: Reversible PAR-1 antagonist, inhibits thrombin- and TRAP-induced platelet aggregation. Reduces thrombotic cardiovascular events in patients with a history of MI or PAD. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:F2R Metabolic genes Substrate:CYP2J2, CYP3A4 Transporter genes:ABCB1 |
|
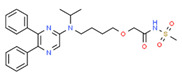
Name: Selexipag IUPAC Name: 2-{4-[(5,6-diphenylpyrazin-2-yl)(propan-2-yl)amino]butoxy}-N-methanesulfonylacetamide. Molecular Formula: C26H32N4O4S Molecular Weight: 496.63 Da Mechanism: Selective PGI2 receptor agonist. Potent vasodilator with antiproliferative, anti-inflammatory, and antithrombotic effects. Effect: Antithrombotic agents. Platelet aggregation inhibitors excl. heparin. |
Mechanistic genes:PTGIR Metabolic genes Substrate:CES1, CYP2C8, CYP3A4 Transporter genes:ABCB1, SLCO1B1, SLCO1B3 |
| Direct thrombin inhibitors | ||

|
Name: Desirudin Molecular Formula: C287H440N80O110S6 Molecular Weight: 6963.52 Da Mechanism: Direct, highly selective thrombin inhibitor. Reversibly binds to the active thrombin site of free and clot-associated thrombin. Inhibits fibrin formation, activation of coagulation factors V, VII, and XIII, and thrombin-induced platelet aggregation. Effect: Antithrombotic agents. Direct thrombin inhibitors. |
Mechanistic genes:F2, F5, F7, F13A1 Metabolic genes Substrate:CPA1 |
|
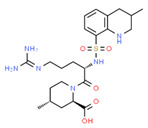
Name:Argatroban IUPAC Name: (1) (2R,4R)-1-{N5-(Diaminomethylene)-N2-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]-L-ornithyl}-4-methyl-2-piperidinecarboxylic acid Molecular Formula: C23H36N6O5S Molecular Weight: 508.634 Da Mechanism: Direct thrombin inhibitor; Reversibly binds to the active thrombin site of free and clot-associated thrombin. Inhibits fibrin formation, activation of coagulation factors V, VIII, and XIII, protein C, and platelet aggregation. Effect: Antithrombotic agents. Direct thrombin inhibitors. |
Mechanistic genes:F2, F5, F8, F13, PROC Metabolic genes Substrate:CYP3A4, CYP3A5 |
|
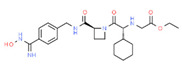
Name: Ximelagatran IUPAC Name: ethyl 2-{[(1R)-1-cyclohexyl-2-[(2S)-2-[({4-[(Z)-N’-hydroxycarbamimidoyl]phenyl}methyl)carbamoyl]azetidin-1-yl]-2-oxoethyl]amino}acetate. Molecular Formula: C24H35N5O5 Molecular Weight: 473.56 Da Mechanism: Bioconverted to the active moiety, melagatran, which inhibits clot-bound thrombin. Effect: Antithrombotic agents. Direct thrombin inhibitors. |
Mechanistic genes:
F2
Metabolic genes Substrate: CYP2C9 |
|
Name:Bivalirudin IUPAC Name: 1) L-Leucine, D-phenylalanyl-L-prolyl-L-arginyl-L-prolylglycylglycylglycylglycyl-L-asparaginylglycyl-L-α-aspartyl-L-phenylalanyl-L-α-glutamyl-L-α-glutamyl-L-isoleucyl-L-prolyl-L-α-glutamyl-L-α-glutamyl-L-tyrosyl-, (2) D-phenylalanyl-L-prolyl-L-arginyl-L-prolylglycylglycylglycylglycyl-L-asparaginylglycyl-L-α-aspartyl-L-phenylalanyl-L-α-glutamyl-L-α-glutamyl-L-isoleucyl-L-prolyl-L-α-glutamyl-L-α-glutamyl-L-tyrosyl-L-leucine Molecular Formula: C98H138N24O33 Molecular Weight: 2180.29 Da Mechanism: Reversible direct thrombin inhibitor for heparin-induced thrombocytopenia. Inhibits thrombin by binding to its catalytic and anion-binding exosite, preventing thrombin-mediated cleavage of fibrinogen to fibrin, activation of factors V, VIII, and XIII, conversion of fibrinogen to fibrin, and platelet activation and aggregation. Effect: Antithrombotic agents. Direct thrombin inhibitors. |
Mechanistic genes:F2, F5, F8, F13, FGA Metabolic genes Inhibitor:MPO |
|
Name:Dabigatran IUPAC Name: Ethyl 3-[[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl](pyridin-2-yl)amino]propanoate Molecular Formula: C34H41N7O5 Molecular Weight: 627.73 Da Mechanism: Inhibits coagulation by preventing thrombin-mediated effects, including cleavage of fibrinogen to fibrin monomers, activation of factors V, VIII, XI and XIII, and inhibition of thrombin-induced platelet aggregation. Effect: Antithrombotic agents. Direct thrombin inhibitors. |
Mechanistic genes:F2, F5, F8, F11, F13, FGA Metabolic genes Substrate:CES1 Transporter genes:ABCB1 |
| Direct factor Xa inhibitors | ||
|
Name: Rivaroxaban IUPAC Name: 5-chloro-N-{[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl}thiophene-2-carboxamide. Molecular Formula: C19H18ClN3O5S Molecular Weight: 435.88 Da Mechanism: Anticoagulant, irreversibly inhibits free and clot bound factor Xa; treating DVT and PE. Effect: Antithrombotic agents. Direct Factor Xa inhibitors. |
Mechanistic genes:F2, F10 Metabolic genes Substrate:CYP2J2, CYP3A4, CYP3A5 Transporter genes:ABCB1, ABCG2 |
|
Name: Apixaban IUPAC Name: 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-1H,4H,5H,6H,7H-pyrazolo[3,4-c]pyridine-3-carboxamide. Molecular Formula: C25H25N5O4 Molecular Weight: 459.50 Da Mechanism: Inhibits factor Xa, independent of antithrombin III. Inhibits prothrombin, preventing thrombus formation. Effect: Antithrombotic agents. Direct Factor Xa inhibitors. |
Mechanistic genes:F2, F5, F10 Metabolic genes Substrate:CYP2C8, CYP2C19, CYP2C9, CYP1A2, CYP2J2, CYP3A4, CYP3A5 Inhibitor:CYP2C19 Transporter genes:ABCB1, ABCG2 |
|
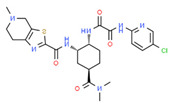
Name: Edoxaban IUPAC Name: N’-(5-chloropyridin-2-yl)-N-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-{5-methyl-4H,5H,6H,7H-[1,3]thiazolo[5,4-c]pyridine-2-amido}cyclohexyl]ethanediamide. Molecular Formula: C24H30ClN7O4S Molecular Weight: 548.06 Da Mechanism: Selective Factor Xa inhibitor. Effect: Antithrombotic agents. Direct Factor Xa inhibitors. |
Mechanistic genes:
F10
Transporter genes: ABCB1 |
|
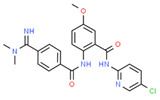
Name: Betrixaban IUPAC Name: N-(5-chloropyridin-2-yl)-2-[4-(N,N-dimethylcarbamimidoyl)benzamido]-5-methoxybenzamide. Molecular Formula: C23H22ClN5O3 Molecular Weight: 451.91 Da Mechanism: Cofactor-independent direct inhibitor of free and prothrombinase-bound Factor Xa. Effect: Antithrombotic agents. Direct Factor Xa inhibitors. |
Mechanistic genes:F2, F10 Transporter genes:ABCB1, KCNH2 |
