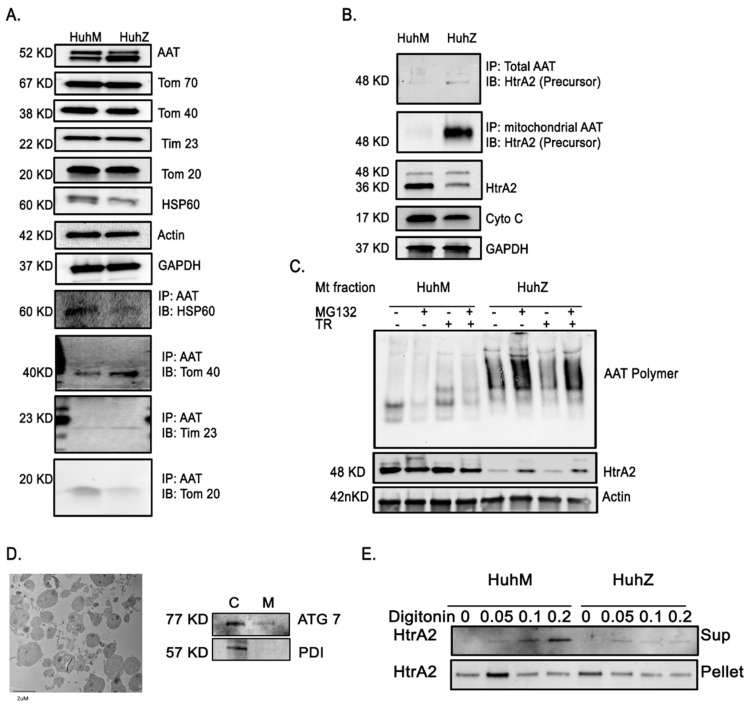
Figure 6.
Mitochondrial interaction of AAT and HtrA2. (A) Detection of AAT translocation to the mitochondria through mitochondrial import machinery using co-immunoprecipitation analysis from total cell lysate, showing both MAAT and ZAAT interaction with Tom40, Tom20, and Tim23 in Huh7.5 cells. (B) Co-immunoprecipitation analysis from total Huh7.5 cell lysate, indicating mitochondrial ZAAT interaction with mitochondrial HtrA2. ZAAT interacts with the full-length precursor form of HtrA2. (C) The aggregation status of mitochondrial AAT and HtrA2 in the mitochondrial fraction of MAAT- and ZAAT-expressing Huh7.5 cells using proteasome inhibitor MG132 and trypsin. Actin was loaded as loading control. (D) Electron microscopy showing mitochondrial fraction purity and Western blot analysis of peroxisomal ATG7 and ER-associated chaperone PDI in cytoplasmic (C) and mitochondrial fraction (M), respectively. (E) Western blot analysis showing the mitochondrial release of HtrA2 in presence of different concentrations of digitonin form MAAT- and ZAAT-expressing hepatocytes.