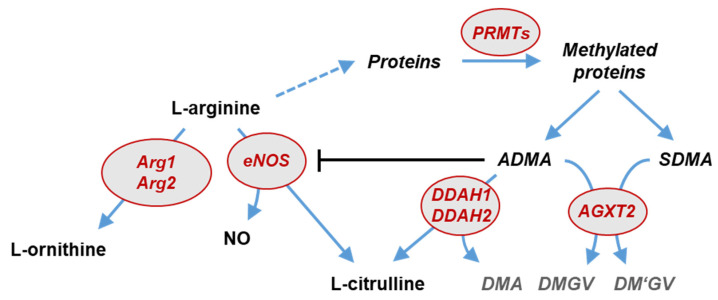
Figure 1.
Schematic representation of the L-arginine—dimethylarginine—nitric oxide pathway. L-arginine is the substrate for endothelial NO synthase and arginases, resulting in the formation of NO and L-citrulline or L-ornithine, respectively. L-arginine residues within specific proteins are subject to methylation by protein arginine N-methyltransferases (PRMTs). After protein hydrolysis, asymmetric (ADMA) and symmetric dimethylarginine (SDMA) are released. ADMA is a competitive inhibitor of nitric oxide synthases (NOS). ADMA, but not SDMA, is degraded by dimethylarginine dimethylaminohydrolases (DDAH1 and DDAH2) into L-citrulline and dimethylamine (DMA). Both dimethylarginines may be cleaved by an alternative pathway through alanine glyoxylate aminotransferase 2 (AGXT2), resulting in the formation of symmetric or asymmetric dimethylguanidinovaleric acid (DMGV and DM’GV). Genes in which single nucleotide polymorphisms were studied in the present study are highlighted in red.