Abstract
The emerging SARS-CoV-2 variants and waning vaccine-elicited immunity are two public health challenges that occurred simultaneously and synergistically during the summer of 2021 and led to a surging demand for COVID-19 vaccine booster dose (BD) rollout. This study aimed to evaluate the COVID-19 vaccine booster hesitancy (VBH) among Czech healthcare workers to explore the potential determinants of VBH. A national cross-sectional survey-based study was carried out between 3 and 11 November 2021, using an online self-administered questionnaire (SAQ) that explored the participants’ demographic characteristics, COVID-19 infection and vaccine anamneses, willingness to receive COVID-19 vaccine BD, and the psychosocial drivers of VBH. A total of 3454 HCW properly responded to the online SAQ, of which 80.9% were females, 30.3% were medical professionals, and 50.5% were ≤47 years old. Most of the participants were already inoculated against SARS-CoV-2 (95.2%), and BTN162b2 was the most commonly administered vaccine (90.7%). As the study sample was planned to represent the target population, it revealed a high level of BD acceptance (71.3%) among Czech HCW, while 12.2% were still hesitant and 16.6% were against the currently available BD. These results are consistent with other recent results from central Europe. Medical professional, male, and older participants were more likely to accept BD rather than allied health professional, female, and younger participants. The BDs’ perceived effectiveness against severe illness, symptomatic infection, and community transmission was a significant and strong predictor for BD acceptance, while the effectiveness against the circulating variants was not that important for our target population. The BDs’ perceived safety and ethical dilemmas of vaccine justice should be addressed sufficiently while communicating with HCW and other population groups. The altruistic reasons for BD acceptance, i.e., family protection, patient protection, and community health protection, underpin the recommendation of postponing the COVID-19 vaccine mandating in favour of stressing these altruistic concerns amid public health messaging.
Keywords: booster immunization, COVID-19 vaccines, Czechia, decision making, health personnel
1. Introduction
Healthcare workers (HCW) have experienced disproportionately high levels of COVID-19-associated morbidity and mortality; therefore, they were prioritized for receiving COVID-19 vaccine booster doses (BDs), which are the third doses of two-dose vaccines, such as BNT162b2 and mRNA-1273, or the second doses of 1-dose vaccines such as Ad26.COV2.S, along with those aged above 65 and the immunocompromised population [1,2]. Since the epidemic flare-ups and effective vaccines’ accessibility are long-standing challenges, distrust of vaccines’ BDs has been increasingly reported, especially in the last few years, triggered by anti-vaccination campaigns [3,4,5].
Recent reports have been increasingly suggesting that the effectiveness of COVID-19 vaccines had declined in several countries within 6 months after the primer doses’ rollout [6,7,8,9]. The recorded epidemic flare-ups and breakthrough cases had been attributed to two possibilities; (a) the emerging variants (mutations) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), or (b) the waning vaccine-elicited immunity that is challenged by the heightened immune evasion by the circulating variants, e.g., the Delta variant, which were not known at the time of the vaccine development and can partially bypass the protective mechanisms established after vaccination and cause illness, leaving the vaccinated cohorts vulnerable [7,8,9,10,11,12]. As both phenomena occur simultaneously and synergistically, the duty of epidemiologic and public health researchers to clarify this foggy scene and provide evidence-informed recommendations regarding the COVID-19 vaccines’ BD timing and priority groups became a challenging one.
It was clearly evident in population-level studies from Scotland, the United States, and Qatar that protection from COVID-19 symptomatic infection can be expected from the two-dose vaccination regimes that were effective against the Delta variant (B.1.617.2) [13,14,15]. Likewise, protection from severe illness can also be achieved according to the population studies of Canada, Scotland, and the United Kingdom, especially against the Alpha variant (B.1.1.7) [9,13,16]. However, the same studies noted a slight drop in the effectiveness of COVID-19 vaccines to around 80%; the sustained reduction in infection risk underscores the continued importance and benefits of COVID-19 vaccination [17]. The good news is that further population studies showed a significant decrease in relative risk of symptomatic infection and severe illness in the patients who received a COVID-19 vaccine BD [18,19]. Therefore, the rollout of BDs -including COVID-19 vaccine BDs- has always been required to battle some of the deadliest diseases, e.g., Hepatitis B and pertussis, which are seen most commonly among HCWs due to their frequent use exposure [20,21].
Vaccine hesitancy (VH) is defined by the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) as “delay in acceptance or refusal of vaccination despite the availability of vaccination services” [22]. COVID-19 vaccine BD hesitancy (VBH) can be predicted in populations with suboptimal vaccine uptake, such as in Poland, where only 51% of the population received a first dose until the middle of September 2021, despite the extensive scientific communication efforts and the worsening epidemic situation in the country in 2021 [23].
Vaccine selectivity (VS) is another emerging public health challenge which can be defined as “the discriminatory attitudes towards certain types of vaccines based on their target contagion or manufacturing technology that yields heterogeneous acceptance levels of recommended vaccines,” and can be simply understood as the individual’s or public’s preference of particular vaccine/s based on their mode of action or brand rather than general preference of immunization [24]. This phenomenon has been commonly seen with childhood vaccines, as parents can be selective with certain vaccines that they believe effective and/or safe for their children, and decline other vaccines [25]. A similar situation had been reportedly experienced with COVID-19 vaccines due to the diversity of vaccine options in many countries, which created a pseudo-competitive ecology for comparing and choosing among the available vaccine brands in the market [26].
An average vaccinee may accept or reject a BD due to various triggers, including; the side effects experienced after the previous (primer) doses, perceived effectiveness of the BD, the vaccinee’s susceptibility to the target infection, and safety uncertainties [23,27]. The WHO-SAGE depicted the perceived effectiveness, safety, and susceptibility and the cost-benefit ratio evaluation as critical determinants for VH, especially for the childhood vaccines which are conventionally recommended worldwide [28,29,30,31].
The safety of COVID-19 vaccines as novel pharmacologic products is a chief priority for health systems that aim to keep tracking them during the post-marketing (phase IV) period through their passive surveillance systems, e.g., VAERS, CAEFISS, and MHRA-Yellow Cards [32,33,34]. In addition, active surveillance studies and hybrid systems had been strongly advocated, especially with the accelerated rollout of COVID-19 vaccines [35,36,37,38]. However, there is still a lack of peer-reviewed evidence on COVID-19 vaccines’ BD side effects; the preliminary findings from passive surveillance systems and the population study of Israel suggest that the BD side effects are not different from the primer doses side effects [18,39,40]. The primer doses were found to induce mild side effects in the vast majority of vaccinees, which lasted for 1–3 days on average in most of COVID-19 vaccines types, i.e., mRNA-based, viral vector-based, and inactivated virus vaccines [24,41,42,43,44,45,46,47,48].
The risk of waning immunity and development of suboptimal immune response after vaccination in older and immunocompromised people, e.g., oncologic patients, organ transplant recipients, and HIV patients, even after two-dose regimens, led to the consensus recommendation of prioritizing these high-risk groups for COVID-19 vaccine BD in various countries, e.g., the United States and France [12,49,50,51,52]. HCWs as a defined high-risk group for COVID-19 infection were also prioritized in all countries that started the rollout of COVID-19 vaccine BDs in late summer 2021, including the Czech Republic [2].
The holistic aim of this study was to evaluate the COVID-19 VBH among HCWs in Czechia. The primary objective was to assess the levels of COVID-19 vaccine BD acceptance and hesitancy among HCWs, and the secondary objective was to explore the potential demographic, anamnestic, and psychosocial determinants of the COVID-19 VBH among the target population.
2. Materials and Methods
2.1. Design
An analytical survey-based cross-sectional study was carried out between 3–11 November 2021 to explore the attitudes of Czech healthcare workers towards receiving the BD (third) doses of COVID-19 vaccines. The study utilized a self-administered questionnaire (SAQ) which was coded and disseminated online through KoBoToolbox (Harvard Humanitarian Initiative, Cambridge, MA, USA, 2021) for data collection from the target participants [53]. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cross-sectional studies governed the design, conduction, and reporting of this study [54].
2.2. Participants
As a nationwide study, the target population (healthcare workers) was approached through various channels, aiming to achieve a nationally representative sample. A non-random technique (snowballing strategy) was used to recruit the participants from all the fourteen regions of the Czech Republic. On 1 November 2021, invitations were sent to the chairs of the professional medical societies, which are members of the Czech Medical Association of J. E. Purkyně (CzMA; Prague, Czech Republic), the member institutions coordinators of the Czech Clinical Research Infrastructure Network (CZECRIN; Brno, Czech Republic), and the inpatient healthcare facilities managers within the network of the Central Adverse Events Reporting System of the Institute of Health Information and Statistics of the Czech Republic (IHIS-CR; Prague, Czech Republic) in order to facilitate participation in the study by circulating the survey uniform resource locator (URL) through their respective networks [55,56,57]. Another channel was used in collaboration with the Ministry of Health to also include healthcare providers for inpatient long-term care; the professional association of non-medical healthcare workers, i.e., Czech Nursing Association (ČAS) [58], Union of Physical Therapists of the Czech Republic (UNIFY-ČR) [59], Chamber of Midwives of the Czech Republic (ČKPA) [60], Association of College Nurses (SVVS) [61], Association of Higher Education Educators in the Non-Medical Health Professions (AVVNZP) [62], and Association of Social Service Providers of the Czech Republic (APSS-ČR) [63]. The study was further promoted through the official websites of professional medical and allied healthcare societies, in addition to the website of the Czech Ministry of Health (MoH) [64].
Epi-Info TM version 7.2.4 (CDC. Atlanta, GA, USA, 2020) was used to calculate the optimal sample size required for this study [65]. The population survey module was run following the assumptions of 2% margin of error, 95% confidence level (CI), 50% outcome probability, and a target population size of 257,118 based on the latest report of IHIS-CR [66,67]. The required sample size was 2379 participants (Supplementary Figure S1).
Participation in this study was entirely voluntary, thus implying that the participants were not forced or rewarded to participate. Additionally, participation in this study was anonymous, to give the participants room to freely express their views and attitudes towards vaccination, aiming to eliminate Hawthorne bias.
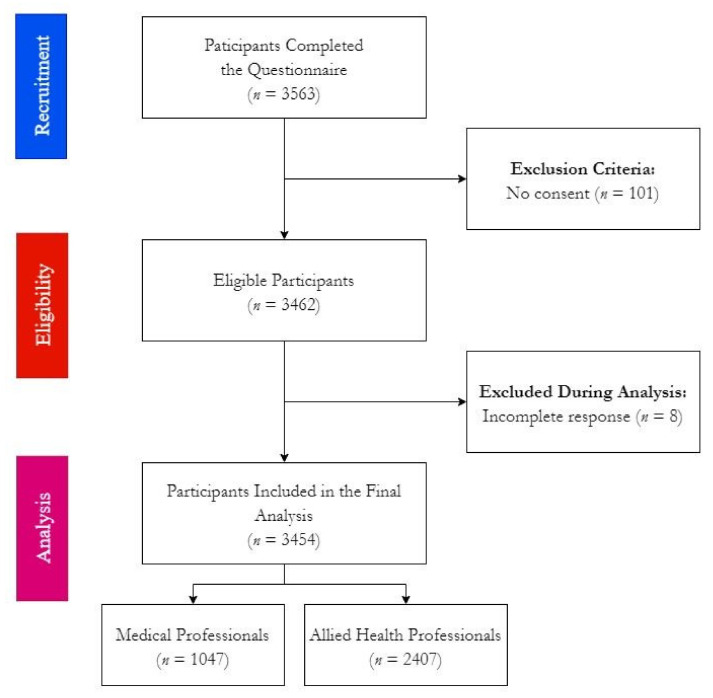
Until 11 November 2021, 3563 responses were received, of which 101 were excluded because the participants did not consent to participate in the study after reading the informed consent, constituting 2.8% as a non-repose rate. Later, eight responses were removed due to incomplete data, leading to a final sample of 3454 participants being included in the final analyses (Figure 1).
Figure 1.
Workflow of COVID-19 Vaccine BD Survey among Czech Healthcare Workers, November 2021 (n = 3454).
2.3. Instrument
The draft SAQ was adapted from previous studies of COVID-19 vaccine hesitancy and consisted of 19 close-ended items stratified into five sections [68,69,70,71,72,73,74,75]. The first section, demographic characteristics, included gender, age, profession, and region. The second section, COVID-19-related anamnesis, included the history of COVID-19 infection, onset, clinical severity, and manifestations of the infection. The third section, COVID-19 vaccine-related anamnesis, included the history of COVID-19 vaccination, vaccine type, and the number of doses. The fourth section included a five-point Likert scale item assessing the willingness to receive BD of COVID-19 vaccines, ranging from “Totally Disagree = 1” to “Totally Agree = 1”.
The fifth section had a set of potential psychosocial drivers of COVID-19 vaccine BD acceptance, including (a) perceived effectiveness: preventing severe illness, symptomatic infection, and community transmission and controlling variants (mutations), (b) perceived safety: equal safety profile compared to primer doses, and seriousness of side effects, (c) perceived susceptibility and risk-benefit ratio, (d) moral dilemma of vaccine justice, and (e) vaccine primer dose satisfaction and vaccine selectivity.
The content validity of the draft SAQ was assessed by a panel of experts in public health medicine, health policy, and healthcare management who provided their feedback on relevance appropriateness and clarity of the proposed items. The experts’ comments guided the development of the pre-test version of the SAQ which was sent to target volunteers who filled it twice with a 14-day interval. The test-re-test reliability of the SAQ yielded a mean Cohen’s kappa coefficient of 0.80 ± 0.19 (IQR: 0.60–1.00), indicating a substantial level of reliability according to McHugh’s criteria [76] (Supplementary Table S1).
2.4. Ethics
The declaration of Helsinki for research involving human subjects guided the design and execution of this study [77]. The study protocol was thoroughly reviewed by the Ethics Committee of the Faculty of Medicine at Masaryk University on 19 October 2021 under the identifier Ref. 63/2021.
All participants had to provide their informed consent as a prerequisite to taking part in the study. The participants were offered to leave the study at any time without justifying their decision; also, no data was saved before the participants finalized the questionnaire and confirmed submitting their answers. No identifying personal data were collected from the participants, in order to keep the study as much anonymous as possible and aiming for the elimination of the Hawthorne bias. The European Union (EU) General Data Protection Regulation (GDPR) was followed during data collection and processing [78].
2.5. Statistics
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 28.0 (SPSS Inc. Chicago, IL, USA, 2020) [79]. Initially, the normal distribution of numerical variables, e.g., age, was tested using the Shapiro-Wilk test. Descriptive statistics were performed to present all the study variables; nominal variables, e.g., gender, profession and discrete events, and ordinal variables, e.g., psychosocial drivers, had been described using frequencies (n) and proportions (%). The numerical variables, e.g., age, had been described using central tendency and dispersion properties.
Consequently, inferential statistics were performed to test for the associations between independent demographic variables (profession, age group, and gender), anamnestic variables (COVID-19 infection and vaccine primer doses), psychosocial drivers, and the dependent variable of BD-related attitudes using the Chi-squared test (χ2) and Fisher’s exact test for tests with <5 predicted frequency. Eventually, univariate logistic regression was performed to evaluate the odds ratio of vaccine hesitancy vs. acceptance for each significant demographic variable. Multivariate regression analysis of the proposed psychosocial drivers was adjusted for gender, pregnancy, age, profession, COVID-19 infection and vaccination, and seeking medical care. All inferential tests were run with the following assumptions; a confidence level (CI) of 95% and significance level (Sig.) of ≤0.05.
3. Results
3.1. Demographic Characteristics
Out of 3454 participants, females were the majority (80.9%), followed by males (18.6%) and LGBTQ+ (0.4%). There were 25 pregnant women among the participating females, of which 36% were in the first trimester, 40% the second trimester, and 24% the third trimester. The mean age of the participants was 46.97 ± 11.78 (IQR: 39–55) years old, and the median was 47 years old Table 1.
Table 1.
Demographic Characteristics of Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Gender | Female † | 2796 | 80.9% |
| Male | 643 | 18.6% | |
| LGBTQ+ | 15 | 0.4% | |
| † Pregnancy | Yes ‡ | 25 | 0.9% |
| No | 2771 | 99.1% | |
| ‡ Trimester | 1st Trimester | 9 | 36% |
| 2nd Trimester | 10 | 40% | |
| 3rd Trimester | 6 | 24% | |
| Age | ≤47 years-old | 1744 | 50.5% |
| >47 years-old | 1710 | 49.5% | |
| Profession | Medical Professionals (MP) | 1047 | 30.3% |
| Allied Health Professionals (AHP) | 2407 | 69.7% |
No missing data. † Female participants. ‡ Pregnant participants.
According to Czech law, Act no. 95/2004 Coll. and Act no. 96/2004 Coll., medical professions (MP) include general medicine, dentistry, and pharmacy, while allied health professions (AHP) include all the non-medical professions which are related to the provision of health care, e.g., nursing, midwifery, and physiotherapy [80,81,82]. Physicians were the most common MP (28.4%), followed by pharmacists (1.5%) and dentists (0.4%). General nurses were the most common AHP (42%), followed by paediatric nurses (4.3%), medical laboratory technicians (4.2%), and pharmaceutical assistants (3.0%). In total, MP represented 30.3% of the participating sample, while AHP represented 69.7% (Supplementary Table S1).
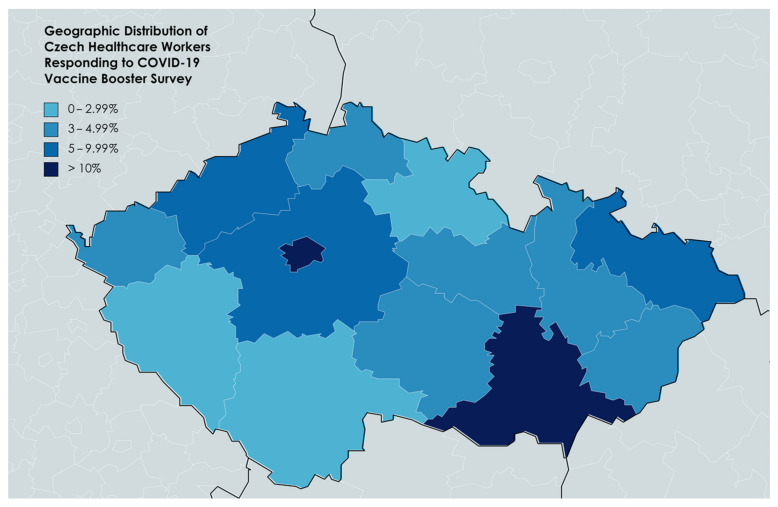
The participating sample represented all the fourteen administrative regions of the Czech Republic, Act no. 129/2000 Coll. [83]. The most contributing region was the capital city Prague (29.2%), followed by the South Moravian (20.2%), the Central Bohemian (9.8%), the Moravian-Silesian (6.0%), and the Ústecký regions (5.4%), shown in Figure 2.
Figure 2.
Geographic Distribution of Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
3.2. COVID-19-Related Anamnesis
In total, 32% of the participants reported being previously infected with COVID-19, with a significant difference between MP (28.4%) vs. AHP (33.6%) and >47 years-old (29.5%) vs. ≤47 years-old (34.5%). Most infections (87.8%) occurred before receiving the first COVID-19 vaccine dose, 6.2% between the first and the second doses, and 6.0% after the second dose. According to the Australian guidelines for the clinical care of people with COVID-19, mild cases were the majority (59.5%), followed by moderate (31.4%) and asymptomatic (7.1%) [84]. There was no statistically significant difference in COVID-19 onset or severity across the profession, gender, or age group.
The most commonly reported clinical manifestation of COVID-19 infection was fatigue (77.1%), followed by myalgia (66.2%), headache (63.3%), anosmia (59.9%), fever (56.4%), cough (49.9%), and dysgeusia (46.2%). Dyspnea was significantly more common among females (30.6%) and AHP (32.8%) compared to males (21.0%) and MP (18.5%). Neurological disorders, i.e., anosmia and dysgeusia, were significantly more common among AHP, females, and the ≤47 year-old group than MP, males, and the >47 year-old group, respectively. Headache, pharyngitis, congestion, and nausea were significantly more common among females than males. Headache, nausea, and vomiting were significantly more common among AHP than MP (Table 2).
Table 2.
COVID-19-related Anamnesis of Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Variable | Outcome | Medical Professionals (n = 1047) |
Allied Health Professionals (n = 2407) |
Sig. | Female (n = 2796) |
Male (n = 643) |
Sig. | ≤47 Years (n = 1744) |
>47 Years (n = 1710) |
Sig. | Total (n = 3454) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection | Yes † | 297 (28.4%) | 809 (33.6%) | 0.002 | 908 (32.5%) | 195 (30.3%) | 0.293 | 602 (34.5%) | 504 (29.5%) | 0.001 | 1106 (32.0%) |
| No | 750 (71.6%) | 1598 (66.4%) | 1888 (67.5%) | 448 (69.7%) | 1142 (65.5%) | 1206 (70.5%) | 2348 (68.0%) | ||||
| † Onset | Before 1st Dose | 257 (86.5%) | 714 (88.3%) | 0.437 | 793 (87.3%) | 175 (89.7%) | 0.352 | 527 (87.5%) | 444 (88.1%) | 0.779 | 971 (87.8%) |
| Between Doses | 22 (7.4%) | 47 (5.8%) | 0.330 | 57 (6.3%) | 12 (6.2%) | 0.948 | 41 (6.8%) | 28 (5.6%) | 0.390 | 69 (6.2%) | |
| After 2nd Dose | 18 (6.1%) | 48 (5.9%) | 0.937 | 58 (6.4%) | 8 (4.1%) | 0.222 | 34 (5.6%) | 32 (6.3%) | 0.624 | 66 (6.0%) | |
| † Severity | Asymptomatic | 17 (5.7%) | 62 (7.7%) | 0.267 | 61 (6.7%) | 18 (9.2%) | 0.217 | 39 (6.5%) | 40 (7.9%) | 0.348 | 79 (7.1%) |
| Mild | 188 (63.3%) | 470 (58.1%) | 0.118 | 542 (59.7%) | 114 (58.5%) | 0.751 | 392 (65.1%) | 266 (52.8%) | <0.001 | 658 (59.5%) | |
| Moderate | 84 (28.3%) | 263 (32.5%) | 0.179 | 290 (31.9%) | 56 (28.7%) | 0.379 | 164 (27.2%) | 183 (36.3%) | 0.001 | 347 (31.4%) | |
| Severe | 5 (1.7%) | 13 (1.6%) | 1.000 * | 13 (1.4%) | 5 (2.6%) | 0.344 * | 6 (1.0%) | 12 (2.4%) | 0.070 | 18 (1.6%) | |
| Critic | 3 (1.0%) | 1 (0.1%) | 0.062 * | 2 (0.2%) | 2 (0.1%) | 0.146 * | 1 (0.2%) | 3 (0.6%) | 0.336 * | 4 (0.4%) | |
| † Sign & Symptoms |
Fever | 185 (62.3%) | 439 (54.3%) | 0.017 | 502 (55.3%) | 119 (61.0%) | 0.143 | 331 (55.0%) | 293 (58.1%) | 0.292 | 624 (56.4%) |
| Cough | 149 (50.2%) | 403 (49.8%) | 0.917 | 461 (50.8%) | 90 (46.2%) | 0.242 | 293 (48.7%) | 259 (51.4%) | 0.368 | 552 (49.9%) | |
| Dyspnea | 55 (18.5%) | 265 (32.8%) | <0.001 | 278 (30.6%) | 41 (21.0%) | 0.007 | 160 (26.6%) | 160 (31.7%) | 0.059 | 320 (28.9%) | |
| Fatigue | 229 (77.1%) | 624 (77.1%) | 0.992 | 705 (77.6%) | 145 (74.4%) | 0.322 | 458 (76.1%) | 395 (78.4%) | 0.366 | 853 (77.1%) | |
| Myalgia | 194 (65.3%) | 538 (66.5%) | 0.713 | 601 (66.2%) | 128 (65.6%) | 0.883 | 406 (67.4%) | 326 (64.7%) | 0.334 | 732 (66.2%) | |
| Headache | 167 (56.2%) | 533 (65.9%) | 0.003 | 601 (66.2%) | 97 (49.7%) | <0.001 | 394 (65.4%) | 306 (60.7%) | 0.104 | 700 (63.3%) | |
| Anosmia | 163 (54.9%) | 500 (61.8%) | 0.037 | 574 (63.2%) | 87 (44.6%) | <0.001 | 398 (66.1%) | 265 (52.6%) | <0.001 | 663 (59.9%) | |
| Dysgeusia | 115 (38.7%) | 396 (48.9%) | 0.002 | 444 (48.9%) | 65 (33.3%) | <0.001 | 296 (49.2%) | 215 (42.7%) | 0.031 | 511 (46.2%) | |
| Pharyngitis | 54 (18.2%) | 148 (18.3%) | 0.966 | 177 (19.5%) | 25 (12.8%) | 0.029 | 110 (18.3%) | 92 (18.3%) | 0.994 | 202 (18.3%) | |
| Congestion | 104 (35.0%) | 261 (32.3%) | 0.388 | 319 (35.1%) | 46 (23.6%) | 0.002 | 228 (37.9%) | 137 (27.2%) | <0.001 | 365 (33.0%) | |
| Rhinitis | 100 (33.7%) | 256 (31.6%) | 0.523 | 301 (33.1%) | 55 (28.2%) | 0.180 | 208 (34.6%) | 148 (29.4%) | 0.066 | 356 (32.2%) | |
| Nausea | 29 (9.8%) | 128 (15.8%) | 0.011 | 142 (15.6%) | 14 (7.2%) | 0.002 | 74 (12.3%) | 83 (16.5%) | 0.048 | 157 (14.2%) | |
| Vomiting | 6 (2.0%) | 49 (6.1%) | 0.006 | 49 (5.4%) | 6 (3.1%) | 0.177 | 29 (4.8%) | 26 (5.2%) | 0.795 | 55 (5.0%) | |
| Diarrhea | 46 (15.5%) | 159 (19.7%) | 0.114 | 173 (19.1%) | 31 (15.9%) | 0.303 | 96 (15.9%) | 109 (21.6%) | 0.015 | 205 (18.5%) |
Chi-squared test (χ2) and Fisher’s exact test (*) had been used with a significance level (Sig.) ≤ 0.05. † COVID-19 Infection. The significant associations are in bold font.
3.3. COVID-19 Vaccine-Related Anamnesis
The vast majority (95.2%) of the participating sample had received primer doses of COVID-19 vaccines. There were slight differences in vaccine uptake favouring MP, males, and >47 years-old compared to their counterparts. The most reportedly administered vaccine was BTN162b2 (90.7%), followed by mRNA-1273 (5.3%), AZD1222 (2.7%), and Ad26.COV2.S (1.3%). MP (95.1%) and males (92.8%) had significantly more BTN162b2 compared to AHP (88.7%) and females (92.8%). There were no gender or age-based differences in terms of the administered vaccine type.
Less than half of the sample received a third dose by the time of responding to this survey (48.5%), and the rest received either two doses (49.7%) or one dose (1.8%). MP received significantly (Sig. < 0.001) more three doses (60.2%) than AHP (43.3%). Likewise, males received significantly (Sig. < 0.001) more three doses (60.6%) than females (45.8%). Only 2.2% of the total participants reported seeking medical care following the COVID-19 vaccine primer doses; three reported anaphylaxis, 26 lymphadenopathy, 67 myalgia, 67 arthralgia, 58 fatigue, 70 fever, 17 vomiting, and 65 headache (Table 3).
Table 3.
COVID-19 Vaccine-related Anamnesis of Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Variable | Outcome | Medical Professionals (n = 1047) |
Allied Health Professionals (n = 2407) |
Sig. | Female (n = 2796) |
Male (n = 643) |
Sig. | ≤47 Years (n = 1744) |
>47 Years (n = 1710) |
Sig. | Total (n = 3454) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | Yes † | 1012 (96.7%) | 2275 (94.5%) | 0.007 | 2651 (94.8%) | 625 (97.2%) | 0.010 | 1637 (93.9%) | 1650 (96.5%) | <0.001 | 3287 (95.2%) |
| No | 35 (3.3%) | 132 (5.5%) | 145 (5.2%) | 18 (2.8%) | 107 (6.1%) | 60 (3.5%) | 167 (4.8%) | ||||
| † Vaccine Type | BTN162b2 | 962 (95.1%) | 2018 (88.7%) | <0.001 | 2390 (90.2%) | 580 (92.8%) | 0.041 | 1481 (90.5%) | 1499 (90.8%) | 0.710 | 2980 (90.7%) |
| mRNA-1273 | 19 (1.9%) | 156 (6.9%) | <0.001 | 155 (5.8%) | 20 (3.2%) | 0.008 | 100 (6.1%) | 75 (4.5%) | 0.046 | 175 (5.3%) | |
| AZD1222 | 20 (2.0%) | 68 (3.0%) | 0.097 | 70 (2.6%) | 17 (2.7%) | 0.911 | 36 (2.2%) | 52 (3.2%) | 0.091 | 88 (2.7%) | |
| Ad26.COV2.S | 11 (1.1%) | 33 (1.5%) | 0.402 | 36 (1.4%) | 8 (1.3%) | 0.879 | 20 (1.2%) | 24 (1.5%) | 0.561 | 44 (1.3%) | |
| † Doses Number | One | 16 (1.6%) | 43 (1.9%) | 0.539 | 50 (1.5%) | 9 (1.4%) | 0.451 | 29 (1.8%) | 30 (1.8%) | 0.918 | 59 (1.8%) |
| Two | 387 (38.2%) | 1246 (54.7%) | <0.001 | 1388 (52.4%) | 237 (37.9%) | <0.001 | 907 (55.4%) | 726 (44.0%) | <0.001 | 1633 (49.7%) | |
| Three | 609 (60.2%) | 986 (43.3%) | <0.001 | 1213 (45.8%) | 379 (60.6%) | <0.001 | 701 (42.8%) | 894 (54.2%) | <0.001 | 1595 (48.5%) | |
| † Medical Care | Yes | 23 (2.3%) | 90 (4.0%) | 0.014 | 98 (3.7%) | 13 (2.1%) | 0.044 | 64 (3.9%) | 49 (3.0%) | 0.139 | 113 (3.4%) |
| No | 989 (97.7%) | 2185 (96.0%) | 2553 (96.3%) | 612 (97.9%) | 1573 (96.1%) | 1601 (97.0%) | 3174 (96.6%) |
Chi-squared test (χ2) had been used with a significance level (Sig.) ≤ 0.05. † COVID-19 Vaccination. The significant associations are in bold font.
3.4. COVID-19 Vaccine BD-Related Attitudes
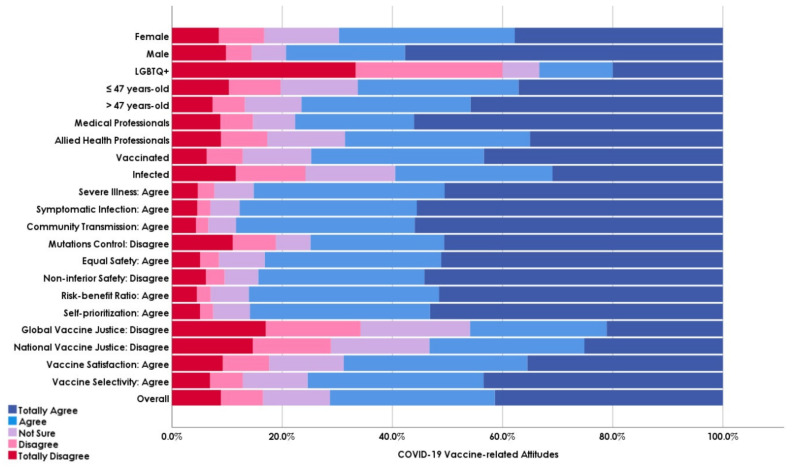
When asked about their attitudes towards receiving COVID-19 vaccine BDs, 71.3% indicated their acceptance (“totally agree” and “agree”), 12.2% were hesitant (“not sure”), and 16.5% disclosed their rejection to receive the BD (“totally disagree” and “disagree”). MP (77.7%), male (79.3%), and >47 year-old participants (76.5%) had significantly (Sig. < 0.001; <0.001; and <0.001) higher levels of BD acceptance than AHP (68.6%), female (69.7%), and ≤47 year-old participants (66.3%), (Figure 3).
Figure 3.
COVID-19 Vaccine BD-related Attitudes of Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
The BD-accepting group was asked about their motivators for receiving the BD; the most common promoter was to protect their families (83.0%), followed by protecting their own health (82.7%), protecting their patients (70.4%), community health protection (66.4%), and having lesser restrictions on the social activities, e.g., travel (49.8%). Employer’s endorsement was reported as the motivator for only 3.4% of the participants.
MP had significantly (Sig. < 0.001; and <0.001) higher levels of interest in patients’ protection and community health protection (74.8% and 76.0%, respectively) compared to AHP (68.2% and 61.7%, respectively). Similarly, male participants had a significantly (Sig. < 0.001) higher level of interest in community health protection (73.7%) than their female counterparts (64.4%), (Table 4).
Table 4.
COVID-19 Vaccine-related Attitudes of Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Variable | Outcome | Medical Professionals (n = 1047) |
Allied Health Professionals (n = 2407) |
Sig. | Female (n = 2796) |
Male (n = 643) |
Sig. | ≤47 Years (n = 1744) |
>47 Years (n = 1710) |
Sig. | Total (n = 3454) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Attitudes | Rejection | 154 (14.7%) | 416 (17.3%) | 0.061 | 468 (16.7%) | 93 (14.5%) | 0.159 | 344 (19.7%) | 226 (13.2%) | <0.001 | 570 (16.5%) |
| Hesitancy | 80 (7.6%) | 340 (14.1%) | <0.001 | 379 (13.6%) | 40 (6.2%) | <0.001 | 244 (14.0%) | 176 (10.3%) | <0.001 | 420 (12.2%) | |
| Acceptance † | 813 (77.7%) | 1651 (68.6%) | <0.001 | 1949 (69.7%) | 510 (79.3%) | <0.001 | 1156 (66.3%) | 1308 (76.5%) | <0.001 | 2464 (71.3%) | |
| † Promoter | Self-protection | 773 (95.1%) | 1511 (91.5%) | 0.001 | 1806 (92.7%) | 473 (92.7%) | 0.949 | 1068 (92.4%) | 1216 (93.0%) | 0.582 | 2284 (82.7%) |
| Patient Prot. | 608 (74.8%) | 1126 (68.2%) | <0.001 | 1376 (70.6%) | 354 (69.4%) | 0.601 | 817 (70.7%) | 917 (70.1%) | 0.758 | 1734 (70.4%) | |
| Family Prot. | 690 (84.9%) | 1354 (82.0%) | 0.076 | 1626 (83.4%) | 414 (81.2%) | 0.229 | 996 (86.2%) | 1048 (80.1%) | <0.001 | 2044 (83.0%) | |
| Community Prot. | 618 (76.0%) | 1018 (61.7%) | <0.001 | 1256 (64.4%) | 376 (73.7%) | <0.001 | 788 (68.2%) | 848 (64.8%) | 0.080 | 1636 (66.4%) | |
| Avoid Testing | 238 (29.3%) | 482 (29.2%) | 0.967 | 561 (28.8%) | 158 (31.0%) | 0.332 | 344 (29.8%) | 376 (28.7%) | 0.582 | 720 (29.2%) | |
| Easier Mobility | 419 (51.5%) | 809 (49.0%) | 0.236 | 959 (49.2%) | 267 (52.4%) | 0.206 | 603 (52.2%) | 625 (47.8%) | 0.030 | 1128 (49.8%) | |
| Employer | 26 (3.2%) | 58 (3.5%) | 0.685 | 61 (3.1%) | 23 (4.5%) | 0.127 | 38 (3.3%) | 46 (3.5%) | 0.754 | 84 (3.4%) |
Chi-squared test (χ2) had been used with a significance level (Sig.) ≤ 0.05. Prot. refers to protection. † COVID-19 Vaccine Booster Accepting-Group. The significant associations are in bold font.
3.5. Psychosocial Drivers of BD-Related Attitudes
The perceived effectiveness of BDs was assessed through four items; (i) severe illness, (ii) symptomatic infection, (iii) community transmission, and (iv) mutation control. A total of 80.3% of the participants agreed that the current BD could protect them from severe illness, while only 57.8% agreed that BDs could prevent symptomatic infection. A total of 60.8% and 65.1% of the participants agreed that BDs could prevent community transmission and tackle the new circulating variants, respectively.
The perceived safety was assessed using two items; (i) equal safety profile and (ii) non-inferior safety (severe side effects). In total, 76.5% agreed that the current BDs are as safe as the primer doses, while only 12.5% believe that the BD may impose more severe side effects compared to the primer ones.
The perceived susceptibility was assessed using one item exploring the participants’ views of self-prioritization for BDs; 67.2% of the participants agreed to be prioritized to receive the currently available BDs, and 78.1% believed that the benefits of BDs outweigh their risks.
The moral dilemma of vaccine justice was evaluated on two levels; (i) globally and (ii) nationally. Less than one-third (30.5%) of the participants disagreed with receiving the BD after learning that administering third doses in developed economies may deprive masses in the third world from getting even the first dose. More than one-third (37.6%) of the participants disagreed with receiving the BD after learning that this may affect the accessibility of some population groups to the vaccine (Table 5).
Table 5.
Drivers of COVID-19 Vaccine-related Attitudes among Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Variable | Outcome | Frequency (n) | Percentage (%) |
|---|---|---|---|
| [Severe Illness] I think the currently available BD (third shots) can protect me from severe COVID-19 infection. | Disagreement | 309 | 8.9% |
| Not Sure | 373 | 10.8% | |
| Agreement | 2772 | 80.3% | |
| [Symptomatic Infection] I think the currently available BD (third shots) can protect me from symptomatic COVID-19 infection. | Disagreement | 663 | 19.2% |
| Not Sure | 793 | 23.0% | |
| Agreement | 1998 | 57.8% | |
| [Community Transmission] I think the currently available BD (third shots) can prevent community transmission of SARS-CoV-2 and its variants. | Disagreement | 645 | 18.7% |
| Not Sure | 709 | 20.5% | |
| Agreement | 2100 | 60.8% | |
| [Mutations Control] I will not take the third shoot (BD) until I find reliable evidence confirming their ability to tackle the new circulating variants of SARS-CoV-2. | Disagreement | 589 | 17.1% |
| Not Sure | 618 | 17.9% | |
| Agreement | 2247 | 65.1% | |
| [Equal Safety] I think the currently available BD (third shots) are as safe as the previous doses of COVID-19 vaccines. | Disagreement | 241 | 7.0% |
| Not Sure | 570 | 16.5% | |
| Agreement | 2643 | 76.5% | |
| [Non-inferior Safety] I think that the currently available BD (third shots) are as safe as the previous doses of COVID-19 vaccines. | Disagreement | 1929 | 55.8% |
| Not Sure | 1094 | 31.7% | |
| Agreement | 431 | 12.5% | |
| [Risk-benefit Ratio] I believe that the benefits of BD (third shots) outweigh their risks. | Disagreement | 331 | 9.6% |
| Not Sure | 426 | 12.3% | |
| Agreement | 2697 | 78.1% | |
| [Self-prioritization] I agree to be prioritized to receive the currently available BD (third shorts). | Disagreement | 625 | 18.1% |
| Not Sure | 509 | 14.7% | |
| Agreement | 2320 | 67.2% | |
| [Global Vaccine Justice] I agree to receive the BD (third shot) of the COVID-19 vaccine even after learning that administering third shots in developed economies may deprive masses in the third world from getting even the first dose. | Disagreement | 1052 | 30.5% |
| Not Sure | 1255 | 36.3% | |
| Agreement | 1147 | 33.2% | |
| [National Vaccine Justice] I agree to receive the BD (third shot) of the COVID-19 vaccine even after learning that this may affect the accessibility of some population groups to the vaccine. | Disagreement | 1297 | 37.6% |
| Not Sure | 1153 | 33.4% | |
| Agreement | 1004 | 29.1% | |
| [Vaccine Satisfaction] I think I should receive a different vaccine type/brand for the BD from the previous doses. | Disagreement | 1405 | 40.7% |
| Not Sure | 1680 | 48.6% | |
| Agreement † | 369 | 10.7% | |
| [Vaccine Selectivity] I think the government should purchase a particular vaccine type/brand for the BD. | Disagreement | 1071 | 31.0% |
| Not Sure | 1644 | 47.6% | |
| Agreement † | 739 | 21.4% | |
| † [Preferred Vaccine] Which vaccine type should be promoted for BD? | BTN162b2 | 611 | 69.3% |
| mRNA-1273 | 193 | 21.9% | |
| AZD1222 | 21 | 2.4% | |
| Ad26.COV2.S | 57 | 6.5% |
No missing data. † Agreement with “Vaccine Satsification” or “Vaccine Selectivity” statements.
Dissatisfaction with the primer doses type was reported by only 10.7% of the participants. More than one-fifth (21.4%) of the participants were vaccine-selective as they agreed that the government should purchase a certain vaccine type/brand for the BD, of which BTN162b2 was the most commonly recommended type (69.3%), followed by mRNA-1273 (21.9%), Ad26.COV2.S (6.5%), and AZD1222 (2.4%).
MP had significantly (Sig. < 0.001, <0.001 and <0.001) higher agreement with the BD capacity against severe illness, symptomatic infection, and community transmission (85.7%, 63.7%, and 66.9%) compared to AHP (77.9%, 55.3%, and 58.2%), respectively. There was no significant (Sig. = 0.808) difference between MP (64.8%) and AHP (65.2%) in terms of agreement with the BD capacity against mutations. MP had significantly (Sig. < 0.001 and <0.001) higher agreement with the equal safety of BDs and higher disagreement with the increased severity of BD side effects (84.5% and 65.8%) compared to AHP (73.0% and 51.5%), respectively.
MP had significantly (Sig. < 0.001, <0.001, and <0.001) higher agreement with the BD capacity against severe illness, symptomatic infection, and community transmission (85.7%, 63.7%, and 66.9%) compared to AHP (77.9%, 55.3%, and 58.2%), respectively. There was no significant (Sig. = 0.808) difference between MP (64.8%) and AHP (65.2%) in terms of agreement with the BD capacity against mutations. MP had significantly (Sig. < 0.001 and <0.001) higher agreement with the equal safety of BDs and higher disagreement with the increased severity of BD side effects (84.5% and 65.8%) compared to AHP (73.0% and 51.5%), respectively.
Moreover, MP had significantly (Sig. < 0.001 and <0.001) higher levels of favourable BD risk-benefit ratio and perceived susceptibility (84.1% and 72.6%) than AHP (75.4% and 64.8%), respectively. Interestingly, MP had significantly (Sig. < 0.001 and <0.001) lower levels of disagreement to receive BDs due to vaccine justice dilemmas globally and nationally (23.3% and 31.5%) compared to AHP (33.6% and 40.2%), respectively. There was no significant difference in terms of primer dose satisfaction or vaccine selectivity between MP and AHP.
Males had significantly (Sig. < 0.001, <0.001, and =0.014) higher agreement with the BD capacity against severe illness, symptomatic infection, and community transmission (86.8%, 65.8%, and 65.2%) compared to females (78.9%, 56.3%, and 59.9%), respectively. There was no significant (Sig. = 0.077) difference between males (62.1%) and females (65.7%) in terms of agreement with the BD capacity against mutations. Males had significantly (Sig. < 0.001 and <0.001) higher agreement with the equal safety of BD and higher disagreement with the increased severity of BD side effects (85.8% and 70.5%) compared to females (74.6% and 52.5%), respectively.
Additionally, males had significantly (Sig. < 0.001 and <0.001) higher levels of favourable BD risk-benefit ratio and perceived susceptibility (85.4% and 72.8%) than females (76.6% and 66.0%), respectively. Likewise, males had significantly (Sig. < 0.001 and <0.001) lower levels of disagreement to receive BD due to vaccine justice dilemmas globally and nationally (23.2% and 29.7%) compared to females (32.0% and 39.3%), respectively. All the differences between the ≤47 year-old group and the >47 year-old group were not statistically significant. Table 6.
Table 6.
Drivers of COVID-19 Vaccine-related Attitudes among Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey Stratified by Profession, Gender, and Age Group, November 2021 (n = 3454).
| Variable | Outcome | Medical Professionals (n = 1047) |
Allied Health Professionals (n = 2407) |
Sig. | Female (n = 2796) |
Male (n = 643) |
Sig. | ≤47 Years (n = 1744) |
>47 Years (n = 1710) |
Sig. |
|---|---|---|---|---|---|---|---|---|---|---|
| Severe Illness | Disagree | 78 (7.4%) | 231 (9.6%) | 0.042 | 261 (9.3%) | 43 (6.7%) | 0.033 | 194 (11.1%) | 115 (6.7%) | <0.001 |
| Not Sure | 72 (6.9%) | 301 (12.5%) | <0.001 | 329 (11.8%) | 42 (6.5%) | <0.001 | 210 (12.0%) | 163 (9.5%) | 0.018 | |
| Agree | 897 (85.7%) | 1875 (77.9%) | <0.001 | 2206 (78.9%) | 558 (86.8%) | <0.001 | 1340 (76.8%) | 1432 (83.7%) | <0.001 | |
|
Symptomatic
Infection |
Disagree | 196 (18.7%) | 467 (19.4%) | 0.640 | 548 (19.6%) | 106 (16.5%) | 0.070 | 397 (22.8%) | 266 (15.6%) | <0.001 |
| Not Sure | 184 (17.6%) | 609 (25.3%) | <0.001 | 675 (24.1%) | 114 (17.7%) | <0.001 | 415 (23.8%) | 378 (22.1%) | 0.238 | |
| Agree | 667 (63.7%) | 1331 (55.3%) | <0.001 | 1573 (56.3%) | 423 (65.8%) | <0.001 | 932 (53.4%) | 1066 (62.3%) | <0.001 | |
|
Community
Transmission |
Disagree | 178 (17.0%) | 467 (19.4%) | 0.096 | 521 (18.6%) | 116 (18.0%) | 0.727 | 400 (22.9%) | 245 (14.3%) | <0.001 |
| Not Sure | 169 (16.1%) | 540 (22.4%) | <0.001 | 599 (21.4%) | 108 (16.8%) | 0.009 | 366 (21.0%) | 343 (20.1%) | 0.500 | |
| Agree | 700 (66.9%) | 1400 (58.2%) | <0.001 | 1676 (59.9%) | 419 (65.2%) | 0.014 | 978 (56.1%) | 1122 (65.6%) | <0.001 | |
| Mutations Control | Disagree | 217 (20.7%) | 372 (15.5%) | <0.001 | 443 (15.8%) | 144 (22.4%) | <0.001 | 329 (18.9%) | 260 (15.2%) | 0.004 |
| Not Sure | 152 (14.5%) | 466 (19.4%) | <0.001 | 515 (18.4%) | 100 (15.6%) | 0.087 | 320 (18.3%) | 298 (17.4%) | 0.480 | |
| Agree | 678 (64.8%) | 1569 (65.2%) | 0.808 | 1838 (65.7%) | 399 (62.1%) | 0.077 | 1095 (62.8%) | 1152 (67.4%) | 0.005 | |
| Equal Safety | Disagree | 68 (6.5%) | 173 (7.2%) | 0.463 | 203 (7.3%) | 33 (5.1%) | 0.054 | 145 (8.3%) | 96 (5.6%) | 0.002 |
| Not Sure | 94 (9.0%) | 476 (19.8%) | <0.001 | 508 (18.2%) | 58 (9.0%) | <0.001 | 311 (17.8%) | 259 (15.1%) | 0.033 | |
| Agree | 885 (84.5%) | 1758 (73.0%) | <0.001 | 2085 (74.6%) | 552 (85.8%) | <0.001 | 1288 (73.9%) | 1355 (79.2%) | <0.001 | |
| Non-inferior Safety | Disagree | 689 (65.8%) | 1240 (51.5%) | <0.001 | 1467 (52.5%) | 453 (70.5%) | <0.001 | 956 (54.8%) | 973 (56.9%) | 0.217 |
| Not Sure | 242 (23.1%) | 852 (35.4%) | <0.001 | 955 (34.2%) | 134 (20.8%) | <0.001 | 555 (31.8%) | 539 (31.5%) | 0.848 | |
| Agree | 116 (11.1%) | 315 (13.1%) | 0.101 | 374 (13.4%) | 56 (8.7%) | 0.001 | 233 (13.4%) | 198 (11.6%) | 0.113 | |
| Risk-benefit Ratio | Disagree | 86 (8.2%) | 245 (10.2%) | 0.071 | 272 (9.7%) | 52 (8.1%) | 0.199 | 198 (11.4%) | 133 (7.8%) | <0.001 |
| Not Sure | 80 (7.6%) | 346 (14.4%) | <0.001 | 383 (13.7%) | 42 (6.5%) | <0.001 | 248 (14.2%) | 178 (10.4%) | <0.001 | |
| Agree | 881 (84.1%) | 1816 (75.4%) | <0.001 | 2141 (76.6%) | 549 (85.4%) | <0.001 | 1298 (74.4%) | 1399 (81.8%) | <0.001 | |
| Self-prioritization | Disagree | 142 (13.6%) | 483 (20.1%) | <0.001 | 537 (19.2%) | 83 (12.9%) | <0.001 | 387 (22.2%) | 238 (13.9%) | <0.001 |
| Not Sure | 145 (13.8%) | 364 (15.1%) | 0.332 | 414 (14.8%) | 92 (14.3%) | 0.747 | 291 (16.7%) | 218 (12.7%) | 0.001 | |
| Agree | 760 (72.6%) | 1560 (64.8%) | <0.001 | 1845 (66.0%) | 468 (72.8%) | <0.001 | 1066 (61.1%) | 1254 (73.3%) | <0.001 | |
|
Global Vaccine
Justice |
Disagree | 244 (23.3%) | 808 (33.6%) | <0.001 | 896 (32.0%) | 149 (23.2%) | <0.001 | 629 (36.1%) | 423 (24.7%) | <0.001 |
| Not Sure | 362 (34.6%) | 893 (37.1%) | 0.156 | 1058 (37.8%) | 193 (30.0%) | <0.001 | 596 (34.2%) | 659 (38.5%) | 0.008 | |
| Agree | 441 (42.1%) | 706 (29.3%) | <0.001 | 842 (30.1%) | 301 (46.8%) | <0.001 | 519 (29.8%) | 628 (36.7%) | <0.001 | |
| National Vaccine Justice | Disagree | 330 (31.5%) | 967 (40.2%) | <0.001 | 1099 (39.3%) | 191 (29.7%) | <0.001 | 766 (43.9%) | 531 (31.1%) | <0.001 |
| Not Sure | 323 (30.9%) | 830 (34.5%) | 0.037 | 961 (34.4%) | 188 (29.2%) | 0.013 | 513 (29.4%) | 640 (37.4%) | <0.001 | |
| Agree | 394 (37.6%) | 610 (25.3%) | <0.001 | 736 (26.3%) | 264 (41.1%) | <0.001 | 465 (26.7%) | 539 (31.5%) | 0.002 | |
|
Vaccine
Satisfaction |
Disagree | 393 (37.5%) | 1012 (42.0%) | 0.013 | 1151 (41.2%) | 248 (38.6%) | 0.227 | 764 (43.8%) | 641 (37.5%) | <0.001 |
| Not Sure | 531 (50.7%) | 1149 (47.7%) | 0.107 | 1370 (49.0%) | 303 (47.1%) | 0.391 | 786 (45.1%) | 894 (52.3%) | <0.001 | |
| Agree | 123 (11.7%) | 246 (10.2%) | 0.182 | 275 (9.8%) | 92 (14.3%) | <0.001 | 194 (11.1%) | 175 (10.2%) | 0.397 | |
|
Vaccine
Selectivity |
Disagree | 304 (29.0%) | 767 (31.9%) | 0.098 | 875 (31.3%) | 190 (29.5%) | 0.388 | 629 (36.1%) | 442 (25.8%) | <0.001 |
| Not Sure | 504 (48.1%) | 1140 (47.4%) | 0.675 | 1346 (48.1%) | 292 (45.4%) | 0.212 | 790 (45.3%) | 854 (49.9%) | 0.006 | |
| Agree | 239 (22.8%) | 500 (20.8%) | 0.176 | 575 (20.6%) | 161 (25.0%) | 0.013 | 325 (18.6%) | 414 (24.2%) | <0.001 | |
|
Preferred
Vaccine |
BTN162b2 | 189 (66.3%) | 422 (70.7%) | 0.188 | 476 (70.0%) | 132 (66.7%) | 0.371 | 257 (63.9%) | 354 (73.8%) | 0.002 |
| mRNA-1273 | 68 (23.9%) | 125 (20.9%) | 0.326 | 147 (21.6%) | 45 (22.7%) | 0.740 | 105 (26.1%) | 88 (18.3%) | 0.005 | |
| AZD1222 | 11 (3.9%) | 10 (1.7%) | 0.047 | 11 (1.6%) | 10 (5.1%) | 0.013 * | 9 (2.2%) | 12 (2.5%) | 0.800 | |
| Ad26.COV2.S | 17 (6.0%) | 40 (6.7%) | 0.678 | 46 (6.8%) | 11 (5.6%) | 0.543 | 31 (7.7%) | 26 (5.4%) | 0.167 |
Chi-squared test (χ2) and Fisher’s exact test (*) had been used with a significance level (Sig.) ≤ 0.05. The significant associations are in bold font.
3.6. Determinants of BD-Related Attitudes
All the demographic variables had impact on BD acceptance; male, non-pregnant women, MP, and >47 year-old participants had significantly (Sig. < 0.001, <0.001, <0.001, and <0.001) higher levels of BD acceptance (79.3%, 70.0%, 76.5%, and 77.7%) compared to female, pregnant women, AHP, and ≤47 year-old participants (69.7%, 36.0%, 66.3%, and 68.6%), respectively.
While the previously infected participants (59.5%) had a significantly (Sig. < 0.001) lower level of BD acceptance compared to their counterparts (76.9%), there was no significant difference due to onset, clinical severity or most of the clinical manifestations.
Regarding the vaccine-related anamnesis, the previously vaccinated participants (74.7%) had a significantly (Sig. < 0.001) higher level of BD acceptance compared to the non-vaccinated participants (4.2%). BTN162b2 was the vaccine type associated with highest level of BD acceptance (76.6%), while Ad26.COV2.S had the lowest level of BD acceptance (27.3%). The participants who sought medical care following their primer dose (38.1%) had a significantly (Sig. < 0.001) lower level of BD acceptance than their counterparts (76.1%).
The agreement with the BD capacity against severe illness, symptomatic infection, and community transmission was significantly associated (Sig. < 0.001, <0.001, and <0.001) with higher levels of BD acceptance (85.2%, 87.7%, and 88.4%) compared to the disagreement with these constructs (11.0%, 39.2%, and 29.6%), respectively. Contrarily, the agreement with the BD capacity against mutations (69.8%) was not significantly different from the disagreement (74.9%). Moreover, the agreement with equal safety and the disagreement with severer side effects were significantly associated (Sig. < 0.001 and <0.001) with BD acceptance (83.2% and 84.3%) compared to the disagreement with equal safety and the agreement with severer side effects (15.4% and 48.3%), respectively.
Likewise, the favourable risk-benefit ratio and agreement with perceived susceptibility were significantly associated (Sig. < 0.001 and <0.001) with higher BD acceptance (86.1% and 85.9%) compared to the unfavourable risk-benefit ratio and disagreement with the perceived susceptibility (16.0% and 33.3%), respectively. The participants who were affected by the ethical dilemmas of vaccine justice globally and nationally were significantly associated (Sig. < 0.001 and <0.001) with decreased levels of BD acceptance (45.9% and 53.3%) compared to the participants who were not affected by the dilemmas (87.7% and 87.6%).
The primer dose satisfaction did not impact the BD acceptance, while the vaccine selectively led to a non-significant increase in BD acceptance. The BD acceptance was the highest in BTN162b2 as the preferred vaccine type (78.1%), and the lowest in the case of Ad26.COV2.S (35.1%) (Table 7).
Table 7.
Determinants of COVID-19 Vaccine-related Attitudes among Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Variable | Outcome | Rejection (n = 570; 16.5%) |
Sig. | Hesitancy (n = 420; 12.2%) |
Sig. | Acceptance (n = 2464; 71.3%) |
Sig. | |
|---|---|---|---|---|---|---|---|---|
| Demographic | Gender | Female † | 468 (16.7%) | 0.159 | 379 (13.6%) | <0.001 | 1949 (69.7%) | <0.001 |
| Male | 93 (14.5%) | 40 (6.2%) | 510 (79.3%) | |||||
| † Pregnancy | Yes | 12 (48.0%) | <0.001 | 4 (16.0%) | 0.720 | 9 (36.0%) | <0.001 | |
| No | 456 (16.5%) | 375 (13.5%) | 1940 (70.0%) | |||||
| Age Group | ≤47 years-old | 344 (19.7%) | <0.001 | 244 (14.0%) | <0.001 | 1156 (66.3%) | <0.001 | |
| >47 years-old | 226 (13.2%) | 176 (10.3%) | 1308 (76.5%) | |||||
| Profession | Medical Professionals | 154 (14.7%) | 0.061 | 80 (7.6%) | <0.001 | 813 (77.7%) | <0.001 | |
| Allied Health Professionals | 416 (17.3%) | 340 (14.1%) | 1651 (68.6%) | |||||
| COVID-19-related Anamnesis | Infection | Yes ‡ | 268 (24.2%) | <0.001 | 180 (16.3%) | <0.001 | 658 (59.5%) | <0.001 |
| No | 302 (12.9%) | 240 (10.2%) | 1806 (76.9%) | |||||
| ‡ Onset | Before 1st Dose | 247 (25.4%) | 0.012 | 155 (16.0%) | 0.451 | 569 (58.6%) | 0.104 | |
| Between Doses | 9 (13.0%) | 0.025 | 16 (23.2%) | 0.108 | 44 (63.8%) | 0.455 | ||
| After 2nd Dose | 12 (18.2%) | 0.237 | 9 (13.6%) | 0.549 | 45 (68.2%) | 0.138 | ||
| ‡ Severity | Asymptomatic | 19 (24.1%) | 0.969 | 9 (11.4%) | 0.222 | 51 (64.6%) | 0.341 | |
| Mild | 163 (24.8%) | 0.611 | 106 (16.1%) | 0.857 | 389 (59.1%) | 0.758 | ||
| Moderate | 81 (23.3%) | 0.641 | 63 (18.2%) | 0.252 | 203 (58.5%) | 0.649 | ||
| Severe | 3 (16.7%) | 0.586 * | 2 (11.1%) | 0.753 * | 13 (72.2%) | 0.267 | ||
| Critical | 2 (50.0%) | 0.249 * | 0 (0%) | 1.000 * | 2 (50.0%) | 1.000 * | ||
| ‡ Signs & Symptoms | Fever | 153 (24.5%) | 0.799 | 94 (15.1%) | 0.215 | 377 (60.4%) | 0.477 | |
| Cough | 133 (24.1%) | 0.915 | 96 (17.4%) | 0.315 | 323 (58.5%) | 0.508 | ||
| Dyspnea | 73 (22.8%) | 0.482 | 53 (16.6%) | 0.869 | 194 (60.6%) | 0.625 | ||
| Fatigue | 210 (24.6%) | 0.581 | 155 (18.2%) | 0.002 | 488 (57.2%) | 0.004 | ||
| Myalgia | 175 (23.9%) | 0.725 | 127 (17.3%) | 0.175 | 430 (58.7%) | 0.477 | ||
| Headache | 176 (25.1%) | 0.353 | 128 (18.3%) | 0.017 | 396 (56.6%) | 0.009 | ||
| Anosmia | 170 (25.6%) | 0.181 | 120 (18.1%) | 0.044 | 373 (56.3%) | 0.007 | ||
| Dysgeusia | 129 (25.2%) | 0.466 | 97 (19.0%) | 0.024 | 285 (55.8%) | 0.019 | ||
| Pharyngitis | 56 (27.7%) | 0.200 | 33 (16.3%) | 0.979 | 113 (55.9%) | 0.255 | ||
| Congestion | 99 (27.1%) | 0.115 | 73 (20.0%) | 0.019 | 193 (52.9%) | 0.002 | ||
| Rhinitis | 102 (28.7%) | 0.018 | 67 (18.8%) | 0.114 | 187 (52.5%) | 0.001 | ||
| Nausea | 36 (22.9%) | 0.681 | 26 (16.6%) | 0.917 | 95 (60.5%) | 0.780 | ||
| Vomiting | 10 (18.2%) | 0.283 | 13 (23.6%) | 0.129 | 32 (58.2%) | 0.839 | ||
| Diarrhea | 43 (21.0%) | 0.228 | 41 (20.0%) | 0.109 | 121 (59.0%) | 0.879 | ||
| Vaccine-related Anamnesis | Vaccinated | Yes Ψ | 422 (12.8%) | <0.001 | 408 (12.4%) | 0.044 | 2457 (74.7%) | <0.001 |
| No | 148 (88.6%) | 12 (7.2%) | 7 (4.2%) | |||||
| Ψ Vaccine Type | BTN162b2 | 349 (11.7%) | <0.001 | 349 (11.7%) | <0.001 | 2282 (76.6%) | <0.001 | |
| mRNA-1273 | 35 (20.0%) | 0.004 | 44 (25.1%) | <0.001 | 96 (54.9%) | <0.001 | ||
| AZD1222 | 11 (12.5%) | 0.923 | 10 (11.4%) | 0.762 | 67 (76.1%) | 0.761 | ||
| Ad26.COV2.S | 27 (61.4%) | <0.001 | 5 (11.4%) | 0.823 | 12 (27.3%) | <0.001 | ||
| Ψ Doses Number | One | 29 (49.2%) | <0.001 | 13 (22.0%) | 0.024 | 17 (28.8%) | <0.001 | |
| Two | 291 (17.8%) | <0.001 | 366 (22.4%) | <0.001 | 976 (59.8%) | <0.001 | ||
| Three | 102 (6.4%) | <0.001 | 29 (1.8%) | <0.001 | 1464 (91.8%) | <0.001 | ||
| Ψ Medical Care | Yes | 48 (42.5%) | <0.001 | 22 (19.5%) | 0.021 | 43 (38.1%) | <0.001 | |
| No | 374 (11.8%) | 386 (12.2%) | 2414 (76.1%) | |||||
| Psychosocial Drivers of COVID-19 Vaccine BD-related Attitudes | Severe Illness | Disagree | 246 (79.6%) | <0.001 | 29 (9.4%) | 0.118 | 34 (11.0%) | <0.001 |
| Not Sure | 111 (29.8%) | <0.001 | 193 (51.7%) | <0.001 | 69 (18.5%) | <0.001 | ||
| Agree | 213 (7.7%) | <0.001 | 198 (7.1%) | <0.001 | 2361 (85.2%) | <0.001 | ||
| Symptomatic Infection |
Disagree | 302 (45.6%) | <0.001 | 101 (15.2%) | 0.007 | 260 (39.2%) | <0.001 | |
| Not Sure | 129 (16.3%) | 0.839 | 213 (26.9%) | <0.001 | 451 (56.9%) | <0.001 | ||
| Agree | 139 (7.0%) | <0.001 | 106 (5.3%) | <0.001 | 1753 (87.7%) | <0.001 | ||
| Community Transmission | Disagree | 330 (51.2%) | <0.001 | 124 (19.2%) | <0.001 | 191 (29.6%) | <0.001 | |
| Not Sure | 102 (14.4%) | 0.089 | 190 (26.8%) | <0.001 | 417 (58.8%) | <0.001 | ||
| Agree | 138 (6.6%) | <0.001 | 106 (5.0%) | <0.001 | 1856 (88.4%) | <0.001 | ||
| Mutations Control | Disagree | 111 (18.8%) | 0.093 | 37 (6.3%) | <0.001 | 441 (74.9%) | 0.037 | |
| Not Sure | 77 (12.5%) | 0.003 | 87 (14.1%) | 0.107 | 454 (73.5%) | 0.197 | ||
| Agree | 382 (17.0%) | 0.282 | 296 (13.2%) | 0.013 | 1569 (69.8%) | 0.007 | ||
| Equal Safety | Disagree | 179 (74.3%) | <0.001 | 25 (10.4%) | 0.379 | 37 (15.4%) | <0.001 | |
| Not Sure | 166 (29.1%) | <0.001 | 175 (30.7%) | <0.001 | 229 (40.2%) | <0.001 | ||
| Agree | 225 (8.5%) | <0.001 | 220 (8.3%) | <0.001 | 2198 (83.2%) | <0.001 | ||
| Non-inferior Safety | Disagree | 184 (9.5%) | <0.001 | 118 (6.1%) | <0.001 | 1627 (84.3%) | <0.001 | |
| Not Sure | 232 (21.2%) | <0.001 | 233 (21.3%) | <0.001 | 629 (57.5%) | <0.001 | ||
| Agree | 154 (35.7%) | <0.001 | 69 (16.0%) | 0.009 | 208 (48.3%) | <0.001 | ||
| Risk-benefit Ratio | Disagree | 251 (75.8%) | <0.001 | 27 (8.2%) | 0.019 | 53 (16.0%) | <0.001 | |
| Not Sure | 130 (30.5%) | <0.001 | 206 (48.4%) | <0.001 | 90 (21.1%) | <0.001 | ||
| Agree | 189 (7.0%) | <0.001 | 187 (6.9%) | <0.001 | 2321 (86.1%) | <0.001 | ||
| Self-prioritization | Disagree | 298 (47.7%) | <0.001 | 119 (19.0%) | <0.001 | 208 (33.3%) | <0.001 | |
| Not Sure | 99 (19.4%) | 0.052 | 146 (28.7%) | <0.001 | 264 (51.9%) | <0.001 | ||
| Agree | 173 (7.5%) | <0.001 | 155 (6.7%) | <0.001 | 1992 (85.9%) | <0.001 | ||
| Global Vaccine Justice |
Disagree | 360 (34.2%) | <0.001 | 209 (19.9%) | <0.001 | 483 (45.9%) | <0.001 | |
| Not Sure | 123 (9.8%) | <0.001 | 157 (12.5%) | 0.634 | 975 (77.7%) | <0.001 | ||
| Agree | 87 (7.6%) | <0.001 | 54 (4.7%) | <0.001 | 1006 (87.7%) | <0.001 | ||
| National Vaccine Justice | Disagree | 374 (28.8%) | <0.001 | 232 (17.9%) | <0.001 | 691 (53.3%) | <0.001 | |
| Not Sure | 124 (10.8%) | <0.001 | 136 (11.8%) | 0.643 | 893 (77.5%) | <0.001 | ||
| Agree | 72 (7.2%) | <0.001 | 52 (5.2%) | <0.001 | 880 (87.6%) | <0.001 | ||
| Vaccine Satisfaction | Disagree | 227 (16.2%) | 0.650 | 158 (11.2%) | 0.173 | 1020 (72.6%) | 0.175 | |
| Not Sure | 278 (16.5%) | 0.945 | 212 (12.6%) | 0.422 | 1190 (70.8%) | 0.524 | ||
| Agree | 65 (17.6%) | 0.542 | 50 (13.6%) | 0.387 | 254 (68.8%) | 0.261 | ||
| Vaccine Selectivity | Disagree | 220 (20.5%) | <0.001 | 130 (12.1%) | 0.979 | 721 (67.3%) | <0.001 | |
| Not Sure | 255 (15.5%) | 0.135 | 203 (12.3%) | 0.747 | 1186 (72.1%) | 0.320 | ||
| Agree | 95 (12.9%) | 0.003 | 87 (11.8%) | 0.716 | 557 (75.4%) | 0.006 | ||
| Preferred Vaccine | BTN162b2 | 69 (11.3%) | <0.001 | 65 (10.6%) | 0.255 | 477 (78.1%) | <0.001 | |
| mRNA-1273 | 22 (11.4%) | 0.248 | 24 (12.4%) | 0.627 | 147 (76.2%) | 0.573 | ||
| AZD1222 | 5 (23.8%) | 0.187 | 2 (9.5%) | 0.779 | 14 (66.7%) | 0.398 | ||
| Ad26.COV2.S | 27 (47.4%) | <0.001 | 10 (17.5%) | 0.135 | 20 (35.1%) | <0.001 |
Chi-squared test (χ2) and Fisher’s exact test (*) had been used with a significance level (Sig.) ≤ 0.05. † Female participants. ‡ Pregnant participants. The significant associations are in bold font.
3.7. Analysis of COVID-19 Vaccine BD Hesitancy vs. Acceptance
Univariate logistic regression was performed to estimate the odds ratio (OR) of BD hesitancy and BD acceptance across the significant demographic and anamnestic predictors. Female participants were 2.36 (CI 95%: 1.69–3.31) times more likely to be BD-hesitant than males, and pregnant women were also 1.22 (CI 95%: 0.42–3.57) more likely to be hesitant compared to non-pregnant women. The young age group, AHP, the previously infected participants, the previously non-vaccinated participants, and the participants who sought medical care were 1.42 (CI 95%: 1.15–1.74), 1.99 (CI 95%: 1.54–2.57), 1.71 (CI 95%: 1.39–2.10), 1.83 (CI 95%: 1.01–3.32), and 1.75 (CI 95%: 1.08–2.82) times more likely to be hesitant compared to their counterparts.
Contrarily, male participants were 1.67 (CI 95%: 1.36–2.05) times more likely to be BD-accepting than females, and non-pregnant women were also 4.15 (CI 95%: 1.83–9.43) more likely to accept BD compared to pregnant women. The old age group, MP, the previously non-infected participants, the previously vaccinated participants, and the participants who did not seek medical care were 1.66 (CI 95%: 1.43–1.92), 1.59 (CI 95%: 1.34–1.88), 2.27 (CI 95%: 1.95–2.65), 67.66 (CI 95%: 31.62–144.81), and 5.17 (CI 95%: 3.51–7.63) times more likely to accept BDs than their counterparts (Table 8).
Table 8.
Regression Analysis of COVID-19 Vaccine Hesitancy vs. Acceptance Demographic and Anamnestic Determinants among Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Hesitancy | Acceptance | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | B (SE) | Wald | OR (CI 95%) | Sig. | Predictor | B (SE) | Wald | OR (CI 95%) | Sig. |
| Female (vs. Male) | 0.86 (0.17) | 24.91 | 2.36 (1.69–3.31) | <0.001 | Male (vs. Female) | 0.51 (0.11) | 23.34 | 1.67 (1.36–2.05) | <0.001 |
| Pregnancy: Yes (vs. No) | 0.20 (0.55) | 0.13 | 1.22 (0.42–3.57) | 0.720 | Pregnancy: No (vs. Yes) | 1.42 (0.42) | 11.55 | 4.15 (1.83–9.43) | <0.001 |
| ≤47 yo (vs. >47 yo) | 0.35 (0.11) | 10.98 | 1.42 (1.15–1.74) | <0.001 | >47 yo (vs. ≤47 yo) | 0.50 (0.08) | 43.63 | 1.66 (1.43–1.92) | <0.001 |
| AHP (vs. Medical) | 0.69 (0.13) | 27.85 | 1.99 (1.54–2.57) | <0.001 | Medical (vs. AHP) | 0.46 (0.09) | 29.01 | 1.59 (1.34–1.88) | <0.001 |
| Infection: Yes (vs. No) | 0.54 (0.11) | 25.38 | 1.71 (1.39–2.10) | <0.001 | Infection: No (vs. Yes) | 0.82 (0.08) | 109.11 | 2.27 (1.95–2.65) | <0.001 |
| Vaccinated: No (vs. Yes) | 0.61 (0.30) | 3.95 | 1.83 (1.01–3.32) | 0.047 | Vaccinated: Yes (vs. No) | 4.22 (0.39) | 228.85 | 67.66 (31.62–144.81) | <0.001 |
| Care: Yes (vs. No) | 0.56 (0.24) | 5.23 | 1.75 (1.08–2.82) | 0.022 | Care: No (vs. Yes) | 1.64 (0.20) | 68.74 | 5.17 (3.51–7.63) | <0.001 |
Binary logistic regression had been used with a significance level (Sig.) ≤ 0.05. AHP refers to Allied Health Professionals. The significant associations are in bold font.
Multivariate logistic regression was performed to estimate the adjusted odds ratio (AOR) of BD acceptance across the various psychosocial predictors while controlling for the significant demographic and anamnestic drivers. The agreement with controlling of severe illness, symptomatic infection, and community transmission was associated with an AOR of 25.55 (CI 95%: 19.45–33.57), 5.81 (CI 95%: 4.78–7.07), and 7.90 (CI 95%: 6.47–9.65) times more likely to be BD-accepting compared to their counterparts, respectively.
The disagreement with controlling mutations and severer side effects was associated with AOR of 1.31 (CI 95%: 1.01–1.69) and 3.97 (CI 95%: 3.28–4.81) times more likely to accept BDs, respectively. The favourable risk-benefit ratio and the perceived susceptibility increased the AOR of BD acceptance with 19.42 (CI 95%: 15.20–24.80) and 7.10 (CI 95%: 5.83–8.63) times, respectively. Being influenced by the ethical dilemma of vaccine justice both globally 0.23 (CI 95%: 0.19–0.28) and nationally 0.32 (CI 95%: 0.27–0.39) was associated with a decreased AOR of BD acceptance. Vaccine satisfaction and selectivity had no significant impact on BD acceptance (Table 9).
Table 9.
Regression Analysis of COVID-19 Vaccine Acceptance Psychosocial Determinants among Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
| Predictor | B (SE) | Wald | AOR (CI 95%) | Sig. |
|---|---|---|---|---|
| Severe Illness: Agree | 3.24 (0.14) | 542.23 | 25.55 (19.45–33.57) | <0.001 |
| Symptomatic Infection: Agree | 1.76 (0.10) | 310.51 | 5.81 (4.78–7.07) | <0.001 |
| Community Transmission: Agree | 2.07 (0.10) | 410.49 | 7.90 (6.47–9.65) | <0.001 |
| Mutations Control: Disagree | 0.27 (0.13) | 4.21 | 1.31 (1.01–1.69) | 0.040 |
| Equal Safety: Agree | 1.99 (0.11) | 350.53 | 7.32 (5.94–9.01) | <0.001 |
| Non-inferior Safety: Disagree | 1.38 (0.10) | 198.93 | 3.97 (3.28–4.81) | <0.001 |
| Risk-benefit Ratio: Agree | 2.97 (0.13) | 565.22 | 19.42 (15.20–24.80) | <0.001 |
| Self-prioritization: Agree | 1.96 (0.10) | 383.86 | 7.10 (5.83–8.63) | <0.001 |
| Global Vaccine Justice: Disagree | −1.46 (0.10) | 226.91 | 0.23 (0.19–0.28) | <0.001 |
| National Vaccine Justice: Disagree | −1.14 (0.09) | 146.96 | 0.32 (0.27–0.39) | <0.001 |
| Vaccine Satisfaction: Agree | 0.06 (0.16) | 0.16 | 1.06 (0.78–1.44) | 0.689 |
| Vaccine Selectivity: Agree | 0.14 (0.11) | 1.49 | 1.15 (0.92–1.44) | 0.223 |
Binary logistic regression had been used with a significance level (Sig.) ≤ 0.05 and adjusted for gender, pregnancy, age, profession, COVID-19 infection and vaccination, and seeking medical care. The significant associations are in bold font.
4. Discussion
This study revealed that a high proportion (71.3%) of the Czech HCW favour the COVID-19 vaccine BD, while 12.2% are still hesitant and 16.6% are against the currently available BD. These results are consistent with what had been recently reported by Rzymski et al., 2021, who found that 71% of the Polish adult population declared their willingness to receive a COVID-19 vaccine BD, while the rest were not in favour of BDs [23]. In Japan, Sugawara et al., 2021 revealed that 89.1% of Japanese medical students were willing to receive the hypothetical BDs of COVID-19 vaccines [85].
The target population of this study was Czech HCW; therefore, the harvested sample was intended to be as representative as possible for the target population. According to a recent report of the Czech Statistical Office (CZSO), around 21.9% of the HCW in the Czech Republic were males, while the vast majority (78.1%) were females in 2019 [86]. Our sample reflected this gender distribution, as 18.6% were males and 80.9% were females. The mean age of Czech HCW according to the CZSO report of 2017 was 46.1 years old, thus corresponding with the mean age of our harvested sample (46.97 ± 11.78) [87]. Regarding the health professions’ categories, the latest report of IHIS-CR revealed that about 20.9% were MP and 79.1% were AHP; therefore, MP was over-represented in our sample (30.3%) compared to AHP (69.7%) [67].
Less than one-third (32%) of our participants had reported being infected by SARS-CoV-2 in the previous time. Until 10 November 2021, 1.8 million accumulated cases of COVID-19 were reported in the Czech Republic, representing 17.2% of the general population, thus making the proportion of infected participants higher than the average population [88]. According to Jarkovsky et al., 2021, around 6.2% of the confirmed COVID-19 patients during the first wave in the Czech Republic were suffering from a severe clinical form of the disease [89]. The proportion of the severe and critical cases in our sample was 2% lower than the reported proportion of accumulated hospitalized cases in the Czech Republic (≈ 7.7%) [90,91]. The vast majority of COVID-19 infection cases occurred before receiving the first dose (87.8%), and the rest occurred after the first dose (6.2%) and after the second dose (6%). Frontline HCW are amongst the high risk groups for COVID-19 infection; therefore, they were recommended to follow strict infection control measures for which they exhibited high levels of compliance in multiple locations where the transmission levels were lowered significantly [92,93,94]. Nevertheless, COVID-19 infections among HCW had been frequently reported both before and after the vaccine rollout with a clear and sustained decline in cases number after mass vaccination [95]. This finding confirms the substantial pooled effectiveness of the approved vaccines in the Czech Republic and the European Union, and it is in agreement with the previous findings of rapid decline in both asymptomatic and symptomatic COVID-19 infections among HCW following vaccine rollout in California [95,96].
Most participants (95.2%) reported being vaccinated against SARS-CoV-2, while 167 declared that they were not vaccinated at all. Gilboa et al., 2021 found that up to 97.1% of the surveyed HCW in Israel were willing to be vaccinated, while the most common reason for noncompliance with COVID-19 vaccination was pregnancy [97]. However, devastating inequalities between MP and AHP were reported at the beginning of the COVID-19 vaccine rollout; for instance, in the US, 75.1% of MP were vaccinated and only 45.6% of the AHP by March 2021; the difference was minor between our participating MP (96.7%) and AHP (94.5%) [98]. Moreover, the higher vaccine uptake rate among male and older participants may confirm what had been previously reported by the COVID-19 vaccine hesitancy studies that attempted to predict the drivers of COVID-19 VH and found that female gender and women of a young age can be determinants of lower vaccine acceptance [68,99,100]. The most commonly administered vaccine among our sample was BTN162b2 (90.7%), which was higher than its proportion among the general Czech population (82.7%) as of 15 November 2021 [101]. The second most common vaccine was mRNA-1273 (5.3%), followed by AZD1222 (2.7%) and Ad26.COV2.S (1.3%), and this exact order was found among the general population: 7.8%, 6.9%, and 2.6%, respectively [101].
Our participants’ most cited reason for accepting BD was family protection (83%), followed by self-protection (82.7%). Rutten et al., 2021 laid down a list of evidence-based strategies for addressing COVID-19 VH by clinical organizations, and one of those strategies was to focus on vaccines as an essential tool to protect one’s own and family health [102]. In a large survey-based study of hospital employees in the United States, the most common reason for accepting COVID-19 vaccination was family protection (86.7%), followed by self-protection (82.9%) [103]. Another recent study in Austria found that self-protection (60.3%) and family protection (55.3%) were the most common reasons for compliance with the COVID-19 guidelines; the investigators concluded that community health protection was also a significant altruistic reason for compliance [104]. The third most cited reason for accepting BD was patient protection (70.4%) which was even higher than community health protection (66.4%). One of the solid arguments for mandating COVID-19 vaccination of HCW is patient protection, thus aiming to fulfil the “do no harm” rule through continuing to provide care for all patients, including COVID-19 patients [105]. On comparing the high level of altruistic reasons for BD acceptance, e.g., family protection (82.7%), patient protection (70.4%), and community health protection (66.4%), to the extremely low level of the non-altruistic reasons, e.g., employer endorsement (3.4%), it becomes more evident that the recommendations of Gur-Arie et al., 2021 of implementing policies strengthening the HCW trust in vaccines and health systems should be the first step before proceeding to consider vaccination mandates [105].
The perceived effectiveness of COVID-19 vaccine BD was a significant and robust predictor of BD acceptance among our participants. The capacity to prevent severe illness, symptomatic infection, and community transmission was associated with an increased AOR of BD acceptance: 25.55, 5.81, and 7.90 times, respectively. The current body of evidence confirms the beliefs of Czech HCW who agreed that COVID-19 BD had capacity against infection-related outcomes; as the 7-day effectiveness of BDs was found to be 93% in reduction of hospitalization, 92% for severe illness, and 81% for COVID-19-related mortality when compared to the recipients of two doses five months ago [106]. Moreover, the adjusted rate ratio (ARR) of symptomatic infection was 11.3 times among the non-booster group vs. the booster group; likewise, the ARR of severe illness was 19.5 times among the non-booster group [40]. Presently, evidence is still lacking and required to verify the capacity of BD against community spread of COVID-19 infection, which might explain why the belief of BD capacity against mutations was not a potent predictor of BD acceptance in our sample.
The perceived safety of COVID-19 vaccine BDs was another significant and strong predictor of BD acceptance. While the assuring evidence on BD side effects is still preliminary and predominantly coming from non-peer-reviewed sources, our participants were inclined to believe that the BDs will have a similar safety profile of the primer doses (79.2%) and no more-severe side effects will emerge following BDs (56.9%). The male participants had higher levels of BD perceived safety compared to their female counterparts; therefore, the gender-based differences of the self-reported side effects following the primer doses could be hypothesized as one of the obstacles for females to accept BD as they were more likely bothered by the post-vaccination side effects [24,43,107,108]. It is yet unclear and requires further investigation why the MP may have higher BD perceived safety levels than the AHP. Gadoth et al., 2021 found that nurses were four times more likely to delay COVID-19 vaccination compared to physicians. Given that this finding was frequently reported with the influenza vaccine, the public health authorities were called upon to take this issue seriously because nurses are more involved in administering vaccines and are placed at the front line with patients [109,110,111,112].
The participants in the ethical conflict of accepting COVID-19 vaccine BDs while millions of people are still unvaccinated worldwide significantly lower odds of BD acceptance. On 12 November 2021, the WHO Director-General described the rollout of BDs in high-income countries (HIC) as a scandalous event [113]. “It makes no sense to give boosters to healthy adults, or to vaccinate children, when health workers, older people and other high-risk groups around the world are still waiting for their first dose,” Dr. Tedros Adhanom Ghebreyesus said [113]. One of the suggested approaches to achieve global vaccine equity is to donate excess doses to low- and middle-income countries (LMIC) by HIC through the COVAX platform, and such an approach can protect both LMIC and HIC [114]. Until 15 November 2021, the WHO-SAGE position from COVID-19 vaccine BD is not in favour of re-vaccinating HCW or the general healthy population, but it is also noteworthy that this recommendation will be re-evaluated in December 2021 [115].
The favourable risk-benefit ratio estimation was a significant and robust predictor of BD acceptance among our participants. The same finding was reported earlier among several population groups, e.g., Czech university students [70], Palestinian healthcare students [71], and American HCW [116], who were more likely to accept COVID-19 vaccine when they had favourable risk-benefit ratio assessment. This fundamental concept of an individual’s risk-benefit ratio assessment has been widely used in vaccine communication and vaccine hesitancy research, as it portrays perceived effectiveness and safety of the vaccine from one side and perceived susceptibility to the infection from another side [117]. Rey et al., 2018 used the risk-benefit balance (RBB) to evaluate VH among French parents, and it was proven as an effective method for explaining the VH of human papillomavirus, hepatitis B virus, measles, and seasonal influenza vaccines [117].
4.1. Strengths
To the best of the authors’ knowledge, this is the first study to evaluate the attitudes of the Czech population towards COVID-19 vaccine BD, and what is more important than estimating the general prevalence of BD acceptance is exploring the potential demographic and anamnestic determinants and the psychosocial drivers of VBH. This study used an anonymous online SAQ that aimed to give the respondents room to express their views towards vaccines without restrictions or feeling judged; therefore, we believe that the Hawthorne bias had been controlled in this design. Third, the harvested sample has an optimal size and decent representativeness, so it reflects several essential characteristics of the target population, e.g., mean age, gender distribution, and professional groups. Finally, the current study shed light on the COVID-19 infection rate, clinical severity stratification, and vaccination rate among Czech HCW.
4.2. Limitations
This study was limited by a number of factors, including (a) lack of information about the administered vaccine type of each dose; (b) lack of information on participants’ general medical anamnesis and BMI; (c) an insufficient number of pregnant women, LGBTQ+, and other minority groups; and (d) insufficient information on the post-vaccination experience of the participants, especially the side-effects of primer doses.
4.3. Implications
The results of this study imply that future research on COVID-19 VBH should explore the role of gender and age on VBH across different population groups. Our study also suggests that public health communication regarding COVID-19 BDs should benefit from the following approaches: (a) highlighting and emphasizing the evidence on BD effectiveness against severe illness, symptomatic infection, and community transmission; (b) highlighting and emphasizing the evidence on BD equal safety with the primer doses; (c) addressing the potential ethical conflicts especially those related vaccine justice; and (d) focusing on alteration of the individual’s risk-benefit ratio of BDs to become more favourable, especially among frontline HCW, e.g., nurses. Using altruistic motivators to induce vaccine uptake among HCW should be prioritized over mandating the vaccines.
5. Conclusions
Within the limitations of this study, a high level of BD acceptance (71.3%) was found among Czech HCW, while 12.2% were still hesitant and 16.6% were against the currently available BDs. These results are consistent with other recent results from central Europe. Medical professionals and male and older participants were more likely to accept BDs rather than allied health professionals and female and younger participants. The BDs’ perceived effectiveness against severe illness, symptomatic infection, and community transmission was found to be a significant and robust predictor for BD acceptance, while the effectiveness against the circulating variants was not that important for our target population. The BDs’ perceived safety and ethical dilemmas of vaccine justice should be addressed sufficiently while communicating with HCW and other population groups. The altruistic reasons for BD acceptance, i.e., family protection, patient protection, and community health protection, underpin the recommendation of postponing the COVID-19 vaccine mandating in favour of stressing these altruistic concerns amid public health messaging.
Acknowledgments
This work is dedicated to the more than thirty thousand fatalities and their families who have fallen victim to COVID-19 in Czechia. The authors would like to thank all the anonymous participants due to their time and effort in sharing their booster doses-related views during this critical time. Also, we would like to thank Jitka Klugarová and Michal Koščík, members of Czech National Centre for Evidence-Based Healthcare and Knowledge Translation, members of Department of Quality of Healthcare, Institute for health information and statistics, and Head Nurse of the Ministry of Health of the Czech Republic Alice Sntrnadová, MBA for their help in validating and promoting the survey. We thank the Chairs of professional medical associations in the Czech Republic, who disseminated the SAQ to their members.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines9121437/s1, Figure S1. The sample size of healthcare workers in Czech Republic—Epi-InfoTM version 7.2.4. Table S1. The results of test re-test reliability. Table S2. Demographic Characteristics of Czech Healthcare Workers Responding to COVID-19 Vaccine BD Survey, November 2021 (n = 3454).
Author Contributions
Conceptualization, M.K. and A.R.; methodology, M.K., A.R. and A.P.; software, A.R.; validation, M.K.; formal analysis, A.R.; investigation, A.P. and M.K.; writing—original draft preparation, A.R. and L.M.; writing—review and editing, A.P. and M.K.; supervision, M.K.; project administration, A.R.; funding acquisition, M.K. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Masaryk University, grant number MUNI/IGA/1543/2020 and MUNI/A/1608/2020. The work of M.K., A.R. and A.P. was supported by the INTER-EXCELLENCE grant number LTC20031—“Towards an International Network for Evidence-based Research in Clinical Health Research in the Czech Republic”.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medicine, Masaryk University Ref. 63/2021 on 19 October 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention (CDC) COVID-19 Vaccine Booster Shots. [(accessed on 16 November 2021)]; Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html.
- 2.Ministerstvo Zdravotnictví (MZCR) Posilovací a Dodatečná Dávka (Booster and Extra Doses) [(accessed on 16 November 2021)]; Available online: https://covid.gov.cz/situace/registrace-na-ockovani/posilovaci-dodatecna-davka.
- 3.Atwell J.E., Salmon D.A. Pertussis Resurgence and Vaccine Uptake: Implications for Reducing Vaccine Hesitancy. Pediatrics. 2014;134:602–604. doi: 10.1542/peds.2014-1883. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta P., Bhattacherjee S., Mukherjee A., Dasgupta S. Vaccine hesitancy for childhood vaccinations in slum areas of Siliguri, India. Indian J. Public Health. 2018;62:253. doi: 10.4103/IJPH.IJPH_397_17. [DOI] [PubMed] [Google Scholar]
- 5.Facciola A., Visalli G., Orlando A., Bertuccio M.P., Spataro P., Squeri R., Picerno I., Di Pietro A. Vaccine hesitancy: An overview on parents’ opinions about vaccination and possible reasons of vaccine refusal. J. Public Health Res. 2019;8:13–18. doi: 10.4081/jphr.2019.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Azman A.S., Lu W., Sun R., Zheng N., Ge S., Deng X., Yang J., Leung D.T., Yu H. Prediction of vaccine efficacy of the Delta variant. medRxiv. 2021 doi: 10.1101/2021.08.26.21262699. [DOI] [Google Scholar]
- 7.Lange B., Gerigk M., Tenenbaum T. Breakthrough Infections in BNT162b2-Vaccinated Health Care Workers. N. Engl. J. Med. 2021;385:1145–1146. doi: 10.1056/NEJMc2108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizrahi B., Lotan R., Kalkstein N., Peretz A., Perez G., Ma M.N., Ben-Tov A., Chodick G., Gazit S., Patalon T. Correlation of SARS-CoV-2 Breakthrough Infections to Time-from-vaccine; Preliminary Study. medRxiv. 2021 doi: 10.1101/2021.07.29.21261317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasreen S., Chung H., He S., Brown K.A., Gubbay J.B., Buchan S.A., Fell D.B., Austin P.C., Schwartz K.L., Sundaram M.E., et al. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada. medRxiv. 2021 doi: 10.1101/2021.06.28.21259420. [DOI] [Google Scholar]
- 10.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning immunity of the BNT162b2 vaccine: A nationwide study from Israel. medRxiv. 2021 doi: 10.1101/2021.08.24.21262423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., Daniels R., Hobson P., Hatipoglu E., Ngai Y., et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals | FDA. [(accessed on 16 November 2021)]; Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
- 13.Sheikh A., McMenamin J., Taylor B., Robertson C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., et al. Six-Month Effectiveness of BNT162B2 mRNA COVID-19 Vaccine in a Large US Integrated Health System: A Retrospective Cohort Study. SSRN Electron. J. 2021 doi: 10.2139/ssrn.3909743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Khatib H.A.A., AlMukdad S., Coyle P., Ayoub H.H., Al Kanaani Z., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. medRxiv. 2021 doi: 10.1101/2021.08.11.21261885. [DOI] [PubMed] [Google Scholar]
- 16.Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021 doi: 10.1101/2021.05.22.21257658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowlkes A., Gaglani M., Groover K., Thiese M.S., Tyner H., Ellingson K. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance—Eight U.S. Locations, December 2020-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., et al. BNT162b2 vaccine booster dose protection: A nationwide study from Israel. medRxiv. 2021 doi: 10.1101/2021.08.27.21262679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva-Cayetano A., Foster W.S., Innocentin S., Belij-Rammerstorfer S., Spencer A.J., Burton O.T., Fra-Bidó S., Le Lee J., Thakur N., Conceicao C., et al. A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med. 2021;2:243–262.e8. doi: 10.1016/j.medj.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams J.L., Christensen C.J., McMahon B.J., Bulkow L.R., Cagle H.H., Mayers J.S., Zanis C.L., Parkinson A.J., Margolis H.S. Evaluation of the response to a booster dose of hepatitis B vaccine in previously immunized healthcare workers. Vaccine. 2001;19:4081–4085. doi: 10.1016/S0264-410X(01)00112-8. [DOI] [PubMed] [Google Scholar]
- 21.Jayasundara D., Sheridan S., Randall D., Campbell P., Edmond K., Liu B., McIntyre P.B., Gidding H.F., Wood J.G. 472Long-term effectiveness of 3-dose primary course and 4-year booster dose of pertussis vaccine in Australia. Int. J. Epidemiol. 2021;50 doi: 10.1093/ije/dyab168.321. [DOI] [Google Scholar]
- 22.MacDonald N.E., Eskola J., Liang X., Chaudhuri M., Dube E., Gellin B., Goldstein S., Larson H., Manzo M.L., Reingold A., et al. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Rzymski P., Poniedziałek B., Fal A. Willingness to Receive the Booster COVID-19 Vaccine Dose in Poland. Vaccines. 2021;9:1286. doi: 10.3390/vaccines9111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klugar M., Riad A., Mekhemar M., Conrad J., Buchbender M., Howaldt H.-P., Attia S. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology. 2021;10:752. doi: 10.3390/biology10080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surgo Ventures Six Ways to Better Understand COVID-19 Vaccine Hesitancy. [(accessed on 17 November 2021)]. Available online: https://surgoventures.medium.com/six-ways-to-better-understand-covid-19-vaccine-hesitancy-3689dfd65b86.
- 26.Berg S. Which COVID-19 Vaccine Should I Get? What to Tell Your Patients. [(accessed on 17 November 2021)]. Available online: https://www.ama-assn.org/delivering-care/public-health/which-covid-19-vaccine-should-i-get-what-tell-your-patients.
- 27.World Economic Forum (WEF) Would You Get a COVID-19 Booster Shot if Offered? [(accessed on 16 November 2021)]. Available online: https://www.weforum.org/agenda/2021/09/covid-19-booster-shot-if-offered/
- 28.Díaz Crescitelli M.E., Ghirotto L., Sisson H., Sarli L., Artioli G., Bassi M.C., Appicciutoli G., Hayter M. A Meta-Synthesis Study of the Key Elements Involved in Childhood Vaccine Hesitancy. Volume 180. Public Health; London, UK: 2020. pp. 38–45. [DOI] [PubMed] [Google Scholar]
- 29.Butler R., MacDonald N.E., Eskola J., Liang X., Chaudhuri M., Dube E., Gellin B., Goldstein S., Larson H., Manzo M.L., et al. Diagnosing the determinants of vaccine hesitancy in specific subgroups: The Guide to Tailoring Immunization Programmes (TIP) Vaccine. 2015;33:4176–4179. doi: 10.1016/j.vaccine.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 30.Strategic Advisory Group of Experts on Immunization (SAGE) Report of the Sage Working Group on Vaccine Hesitancy. SAGE; Thousand Oaks, CA, USA: 2014. [Google Scholar]
- 31.Lane S., MacDonald N.E., Marti M., Dumolard L. Vaccine hesitancy around the globe: Analysis of three years of WHO/UNICEF Joint Reporting Form data-2015–2017. Vaccine. 2018;36:3861–3867. doi: 10.1016/j.vaccine.2018.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Vaccine Adverse Event Reporting System (VAERS) [(accessed on 17 November 2021)]; Available online: https://vaers.hhs.gov/
- 33.Government of Canada (Canada.ca) Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) [(accessed on 17 November 2021)]. Available online: https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html.
- 34.Medicines and Healthcare Products Regulatory Agency (MHRA) Coronavirus (COVID-19) Vaccines Adverse Reactions. [(accessed on 17 November 2021)]; Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions.
- 35.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., Sudre C.H., Nguyen L.H., Drew D.A., Merino J., et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jęśkowiak I., Wiatrak B., Grosman-Dziewiszek P., Szeląg A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines. 2021;9:502. doi: 10.3390/vaccines9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riad A., Schünemann H., Attia S., Peričić T.P., Žuljević M.F., Jürisson M., Kalda R., Lang K., Morankar S., Yesuf E.A., et al. COVID-19 Vaccines Safety Tracking (CoVaST): Protocol of a Multi-Center Prospective Cohort Study for Active Surveillance of COVID-19 Vaccines’ Side Effects. Int. J. Environ. Res. Public Health. 2021;18:7859. doi: 10.3390/ijerph18157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klugar M., Riad A. COVID-19 Vaccines Safety Tracking (CoVaST) [(accessed on 15 July 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04834869.
- 39.Berg S. What Doctors Wish Patients Knew about COVID-19 Caccine Boosters. 2021. [(accessed on 17 November 2021)]. Available online: https://www.ama-assn.org/delivering-care/public-health/what-doctors-wish-patients-knew-about-covid-19-vaccine-boosters.
- 40.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., Mizrahi B., Alroy-Preis S., Ash N., Milo R., et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dziedzic A., Riad A., Attia S., Klugar M., Tanasiewicz M. Self-Reported Adverse Events of COVID-19 Vaccines in Polish Healthcare Workers and Medical Students. Cross-Sectional Study and Pooled Analysis of CoVaST Project Results in Central Europe. J. Clin. Med. 2021;10:5338. doi: 10.3390/jcm10225338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riad A., Pokorná A., Attia S., Klugarová J., Koščík M., Klugar M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021;10:1428. doi: 10.3390/jcm10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riad A., Sağıroğlu D., Üstün B., Pokorná A., Klugarová J., Attia S., Klugar M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021;10:2629. doi: 10.3390/jcm10122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riad A., Hocková B., Kantorová L., Slávik R., Spurná L., Stebel A., Havriľak M., Klugar M. Side Effects of mRNA-Based COVID-19 Vaccine: Nationwide Phase IV Study among Healthcare Workers in Slovakia. Pharmaceuticals. 2021;14:873. doi: 10.3390/ph14090873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alhazmi A., Alamer E., Daws D., Hakami M., Darraj M., Abdelwahab S., Maghfuri A., Algaissi A. Evaluation of Side Effects Associated with COVID-19 Vaccines in Saudi Arabia. Vaccines. 2021;9:674. doi: 10.3390/vaccines9060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riad A., Pokorná A., Klugarová J., Antalová N., Kantorová L., Koščík M., Klugar M. Side Effects of mRNA-Based COVID-19 Vaccines among Young Adults (18–30 Years Old): An Independent Post-Marketing Study. Pharmaceuticals. 2021;14:1049. doi: 10.3390/ph14101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almufty H.B., Mohammed S.A., Abdullah A.M., Merza M.A. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:102207. doi: 10.1016/j.dsx.2021.102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riad A., Pokorná A., Mekhemar M., Conrad J., Klugarová J., Koščík M., Klugar M., Attia S. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines. 2021;9:673. doi: 10.3390/vaccines9060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., Garonzik-Wang J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marion O., Del Bello A., Abravanel F., Couat C., Faguer S., Esposito L., Hebral A.L., Izopet J., Kamar N. Safety and Immunogenicity of Anti-SARS-CoV-2 Messenger RNA Vaccines in Recipients of Solid Organ Transplants. Ann. Intern. Med. 2021;174:1336–1338. doi: 10.7326/M21-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., del Molino del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.KoBoToolbox.org KoBoToolbox. [(accessed on 15 August 2020)]. Available online: https://support.kobotoolbox.org/welcome.html.
- 54.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. UroToday Int. J. 2007;335:806–808. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Česká Lékařská Společnost Jana Evangelisty Purkyně (CzMA) Společnosti ČLS JEP, z.s (Member Societies of the Czech Medical Association) [(accessed on 15 November 2021)]. Available online: https://www.cls.cz/spolecnosti-cls-jep.
- 56.CZECRIN Czech Clinical Research Infrastructure Network. [(accessed on 2 March 2021)]. Available online: https://czecrin.cz/en/home/
- 57.Ústav Zdravotnických Informací a Statistiky České Republiky (ÚZIS ČR) Systém Hlášení Nežádoucích Událostí (SHNU) [(accessed on 8 March 2021)]. Available online: https://shnu.uzis.cz/
- 58.Česká Asociace Sester (CNNA) Postoje Zdravotnických Pracovníků ke Třetím (Posilovacím) Dávkám Očkování Proti COVID-19. [(accessed on 15 November 2021)]. Available online: https://www.cnna.cz/
- 59.Nás|UNIFY ČR. [(accessed on 17 November 2021)]. Available online: http://www.unify-cr.cz/o-nas.
- 60.ČKPA—Česká Komora Porodních Asistentek z.s. [(accessed on 17 November 2021)]. Available online: https://www.ckpa.cz/
- 61.SVVS—Kdo Jsme. [(accessed on 17 November 2021)]. Available online: https://www.svvs.cz/
- 62.Avvnzp. [(accessed on 17 November 2021)]. Available online: https://avvnzp.webnode.cz/
- 63.Dokumenty APSS ČR—Asociace|Asociace Poskytovatelů Sociálních Služeb České Republiky. [(accessed on 17 November 2021)]. Available online: https://www.apsscr.cz/cz/asociace/dokumenty-apss-cr.
- 64.Ministerstvo zdravotnictví České republiky (MZČR) Dotazníkový Průzkum: Postoje Zdravotnických Pracovníků ke Třetím (Posilovacím) Dávkám Očkování Proti COVID-19. [(accessed on 15 November 2021)]; Available online: https://www.mzcr.cz/dotaznik-postoje-zdravotnickych-pracovniku-treti-davka-ockovani-covid-19/
- 65.Centers for Disease Control and Prevention (CDC) Epi Info™ for Windows. [(accessed on 25 December 2020)]; Available online: https://www.cdc.gov/epiinfo/pc.html.
- 66.Centers for Disease Control and Prevention (CDC) Population Survey or Descriptive Study. [(accessed on 19 May 2021)]; Available online: https://www.cdc.gov/epiinfo/user-guide/statcalc/samplesize.html.
- 67.Institute of Health Information and Statistics of the Czech Republic (UZIS) Health Yearbook of the Czech Republic 2017. [(accessed on 3 March 2021)]. Available online: https://www.uzis.cz/index-en.php?pg=record&id=8166.
- 68.Riad A., Abdulqader H., Morgado M., Domnori S., Koščík M., Mendes J.J., Klugar M., Kateeb E. Global Prevalence and Drivers of Dental Students’ COVID-19 Vaccine Hesitancy. Vaccines. 2021;9:566. doi: 10.3390/vaccines9060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Riad A., Huang Y., Abdulqader H., Morgado M., Domnori S., Koščík M., Mendes J.J., Klugar M., Kateeb E. IADS-SCORE Universal Predictors of Dental Students’ Attitudes towards COVID-19 Vaccination: Machine Learning-Based Approach. Vaccines. 2021;9:1158. doi: 10.3390/vaccines9101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riad A., Pokorná A., Antalová N., Krobot M., Zviadadze N., Serdiuk I., Koščík M., Klugar M. Prevalence and Drivers of COVID-19 Vaccine Hesitancy among Czech University Students: National Cross-Sectional Study. Vaccines. 2021;9:948. doi: 10.3390/vaccines9090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kateeb E., Danadneh M., Pokorná A., Klugarová J., Abdulqader H., Klugar M., Riad A. Predictors of Willingness to Receive COVID-19 Vaccine: Cross-Sectional Study of Palestinian Dental Students. Vaccines. 2021;9:954. doi: 10.3390/vaccines9090954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Štěpánek L., Janošíková M., Nakládalová M., Štěpánek L., Boriková A., Vildová H. Motivation to COVID-19 Vaccination and Reasons for Hesitancy in Employees of a Czech Tertiary Care Hospital: A Cross-Sectional Survey. Vaccines. 2021;9:863. doi: 10.3390/vaccines9080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trent M., Seale H., Chughtai A., Salmon D., MacIntyre C. Trust in government, intention to vaccinate and COVID-19 vaccine hesitancy: A comparative survey of five large cities in the United States, United Kingdom, and Australia. Vaccine. 2021 doi: 10.1016/j.vaccine.2021.06.048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy J., Vallières F., Bentall R.P., Shevlin M., McBride O., Hartman T.K., McKay R., Bennett K., Mason L., Gibson-Miller J., et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021;12:29. doi: 10.1038/s41467-020-20226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salerno L., Craxì L., Amodio E., Lo Coco G. Factors Affecting Hesitancy to mRNA and Viral Vector COVID-19 Vaccines among College Students in Italy. Vaccines. 2021;9:927. doi: 10.3390/vaccines9080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McHugh M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.(WMA) W.M.A. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA J. Am. Med. Assoc. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 78.Proton Technologies AG General Data Protection Regulation (GDPR) Compliance Guidelines. [(accessed on 1 May 2020)]. Available online: https://gdpr.eu/
- 79.SPSS Inc IBM SPSS Statistics 28. [(accessed on 14 March 2021)]. Available online: https://www.ibm.com/support/pages/ibm-spss-statistics-28-documentation.
- 80.AION-CS 95/2004 Sb. Zákon o Podmínkách Získávání a Uznávání Způsobilosti k Výkonu Povolání Lékařů a Farmaceutů. [(accessed on 15 November 2021)]. Available online: https://www.zakonyprolidi.cz/cs/2004-95.
- 81.AION-CS 96/2004 Sb. Zákon o Nelékařských Zdravotnických Povoláních. [(accessed on 15 November 2021)]. Available online: https://www.zakonyprolidi.cz/cs/2004-96.
- 82.Ústav Zdravotnických Informací a Statistiky ČR (ÚZIS) Rozlišení Lékařských a Nelékařských Zdravotnických Povolání: Lékař, Sestra a Další Nelékařská Povolání. [(accessed on 15 November 2021)]. Available online: https://www.nzip.cz/clanek/479-lekarska-vs-nelekarska-zdravotnicka-povolani.
- 83.AION-CS 129/2000 Sb. Zákon o Krajích. [(accessed on 15 November 2021)]. Available online: https://www.zakonyprolidi.cz/cs/2000-129.
- 84.NHMRC N.H., M.R.C Australian Guidelines for the Clinical Care of People with COVID-19. [(accessed on 17 September 2020)]; Available online: https://www.clinicalguidelines.gov.au/register/australian-guidelines-clinical-care-people-covid-19.
- 85.Sugawara N., Yasui-Furukori N., Fukushima A., Shimoda K., Diclemente J. Attitudes of Medical Students toward COVID-19 Vaccination: Who Is Willing to Receive a Third Dose of the Vaccine? Vaccines. 2021;9:1295. doi: 10.3390/vaccines9111295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Czech Statistical Office (CZSO) Zaostřeno na Ženy a Muže 2018 (Focus on Women and Men 2018) [(accessed on 17 November 2021)]. Available online: https://www.czso.cz/documents/10180/60622084/30000218.pdf.
- 87.Czech Statistical Office (CZSO) Odměňování Zdravotnických Pracovníků (Remuneration of Healthcare Staff) [(accessed on 17 November 2021)]. Available online: https://www.czso.cz/documents/10180/112643651/260024-19.pdf.
- 88.Worldmeter Coronavirus Update (Live) [(accessed on 17 November 2021)]. Available online: https://www.worldometers.info/coronavirus/
- 89.Jarkovsky J., Benesova K., Cerny V., Razova J., Kala P., Dolina J., Majek O., Sebestova S., Bezdekova M., Melicharova H., et al. Covidogram as a simple tool for predicting severe course of COVID-19: Population-based study. BMJ Open. 2021;11:e045442. doi: 10.1136/bmjopen-2020-045442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Komenda M., Bulhart V., Karolyi M., Jarkovský J., Mužík J., Májek O., Šnajdrová L., Růžičková P., Rážová J., Prymula R., et al. Complex reporting of the COVID-19 epidemic in the czech republic: Use of an interactive web-based app in practice. J. Med. Internet Res. 2020;22:e19367. doi: 10.2196/19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.COVID-19 v ČR: Otevřené Datové Sady a Sady ke Stažení | Onemocnění Aktuálně od MZČR. [(accessed on 17 November 2021)]. Available online: https://onemocneni-aktualne.mzcr.cz/api/v2/covid-19.
- 92.Shatnawi N.J., Mesmar Z., Al-Omari G.A., AL-Sheyab W., AlZoubi N.A., AL-Ghazo M., Hamouri S., AL-Faori I., Bani-Essa A., Matalka I., et al. Compliance with safety measures and risk of COVID-19 transmission among healthcare workers. Future Sci. OA. 2021 doi: 10.2144/fsoa-2021-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lai X., Wang X., Yang Q., Xu X., Tang Y., Liu C., Tan L., Lai R., Wang H., Zhang X., et al. Will healthcare workers improve infection prevention and control behaviors as COVID-19 risk emerges and increases, in China? Antimicrob. Resist. Infect. Control. 2020;9:83. doi: 10.1186/s13756-020-00746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ashinyo M.E., Dubik S.D., Duti V., Amegah K.E., Ashinyo A., Asare B.A., Ackon A.A., Akoriyea S.K., Kuma-Aboagye P. Infection prevention and control compliance among exposed healthcare workers in COVID-19 treatment centers in Ghana: A descriptive cross-sectional study. PLoS ONE. 2021;16:e0248282. doi: 10.1371/journal.pone.0248282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Keehner J., Horton L.E., Pfeffer M.A., Longhurst C.A., Schooley R.T., Currier J.S., Abeles S.R., Torriani F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021;384:1774–1775. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gohil S.K., Olenslager K., Quan K.A., Dastur C.K., Afsar N., Chang W., Huang S.S. Asymptomatic and Symptomatic COVID-19 Infections Among Health Care Personnel Before and After Vaccination. JAMA Netw. Open. 2021;4:e2115980. doi: 10.1001/jamanetworkopen.2021.15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilboa M., Tal I., Levin E.G., Segal S., Belkin A., Zilberman-Daniels T., Biber A., Rubin C., Rahav G., Regev-Yochay G. Coronavirus disease 2019 (COVID-19) vaccination uptake among healthcare workers. Infect. Control Hosp. Epidemiol. 2021:1–6. doi: 10.1017/ice.2021.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee J.T., Althomsons S.P., Wu H., Budnitz D.S., Kalayil E.J., Lindley M.C., Pingali C., Bridges C.B., Geller A.I., Fiebelkorn A.P., et al. Disparities in COVID-19 Vaccination Coverage Among Health Care Personnel Working in Long-Term Care Facilities, by Job Category, National Healthcare Safety Network—United States, March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1036–1039. doi: 10.15585/mmwr.mm7030a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sallam M. Covid-19 vaccine hesitancy worldwide: A concise systematic review of vaccine acceptance rates. Vaccines. 2021;9:160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solís Arce J.S., Warren S.S., Meriggi N.F., Scacco A., McMurry N., Voors M., Syunyaev G., Malik A.A., Aboutajdine S., Adeojo O., et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021;27:1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ministerstvo Zdravotnictví ČR (MZČR) COVID-19: Přehled Vykázaných Očkování v ČR. [(accessed on 10 November 2021)]. Available online: https://onemocneni-aktualne.mzcr.cz/vakcinace-cr.
- 102.Finney Rutten L.J., Zhu X., Leppin A.L., Ridgeway J.L., Swift M.D., Griffin J.M., St. Sauver J.L., Virk A., Jacobson R.M. Evidence-Based Strategies for Clinical Organizations to Address COVID-19 Vaccine Hesitancy. Mayo Clin. Proc. 2021;96:699–707. doi: 10.1016/j.mayocp.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuter B.J., Browne S., Momplaisir F.M., Feemster K.A., Shen A.K., Green-McKenzie J., Faig W., Offit P.A. Perspectives on the receipt of a COVID-19 vaccine: A survey of employees in two large hospitals in Philadelphia. Vaccine. 2021;39:1693–1700. doi: 10.1016/j.vaccine.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schernhammer E., Weitzer J., Laubichler M.D., Birmann B.M., Bertau M., Zenk L., Caniglia G., Jäger C.C., Steiner G. Correlates of COVID-19 vaccine hesitancy in Austria: Trust and the government. J. Public Health. 2021:fdab122. doi: 10.1093/pubmed/fdab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gur-Arie R., Jamrozik E., Kingori P. No Jab, No Job? Ethical Issues in Mandatory COVID-19 Vaccination of Healthcare Personnel. BMJ Glob. Health. 2021;6:e004877. doi: 10.1136/bmjgh-2020-004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barda N., Dagan N., Cohen C., Hernán M.A., Lipsitch M., Kohane I.S., Reis B.Y., Balicer R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 108.Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gadoth A., Halbrook M., Martin-Blais R., Gray A., Tobin N.H., Ferbas K.G., Aldrovandi G.M., Rimoin A.W. Cross-sectional Assessment of COVID-19 Vaccine Acceptance Among Health Care Workers in Los Angeles. Ann. Intern. Med. 2021;174:882–885. doi: 10.7326/M20-7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keske Ş., Mutters N.T., Tsioutis C., Ergönül Ö. Influenza vaccination among infection control teams: A EUCIC survey prior to COVID-19 pandemic. Vaccine. 2020;38:8357–8361. doi: 10.1016/j.vaccine.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Hollmeyer H.G., Hayden F., Poland G., Buchholz U. Influenza vaccination of health care workers in hospitals--a review of studies on attitudes and predictors. Vaccine. 2009;27:3935–3944. doi: 10.1016/j.vaccine.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 112.Pless A., McLennan S.R., Nicca D., Shaw D.M., Elger B.S. Reasons why nurses decline influenza vaccination: A qualitative study. BMC Nurs. 2017;16:20. doi: 10.1186/s12912-017-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.World Health Organization (WHO) Media Briefing on COVID-19. [(accessed on 17 November 2021)]. Available online: https://www.youtube.com/watch?v=L3fNLTJT4GU&t=3s&ab_channel=WorldHealthOrganization%28WHO%29.
- 114.Hotez P.J., Batista C., Amor Y.B., Ergonul O., Figueroa J.P., Gilbert S., Gursel M., Hassanain M., Kang G., Kaslow D.C., et al. Global public health security and justice for vaccines and therapeutics in the COVID-19 pandemic. EClinicalMedicine. 2021;39:101053. doi: 10.1016/j.eclinm.2021.101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.WHO-SAGE Interim Statement on Booster Doses for COVID-19 Vaccination. [(accessed on 17 November 2021)]. Available online: https://www.who.int/news/item/04-10-2021-interim-statement-on-booster-doses-for-covid-19-vaccination.
- 116.Parente D.J., Ojo A., Gurley T., Le Master J.W., Meyer M., Wild D.M., Mustafa R.A. Acceptance of COVID-19 Vaccination Among Health System Personnel. J. Am. Board Fam. Med. 2021;34:498–508. doi: 10.3122/jabfm.2021.03.200541. [DOI] [PubMed] [Google Scholar]
- 117.Rey D., Fressard L., Cortaredona S., Bocquier A., Gautier A., Peretti-Watel P., Verger P. Vaccine hesitancy in the French population in 2016, and its association with vaccine uptake and perceived vaccine risk–benefit balance. Eurosurveillance. 2018;23:17-00816. doi: 10.2807/1560-7917.ES.2018.23.17.17-00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.