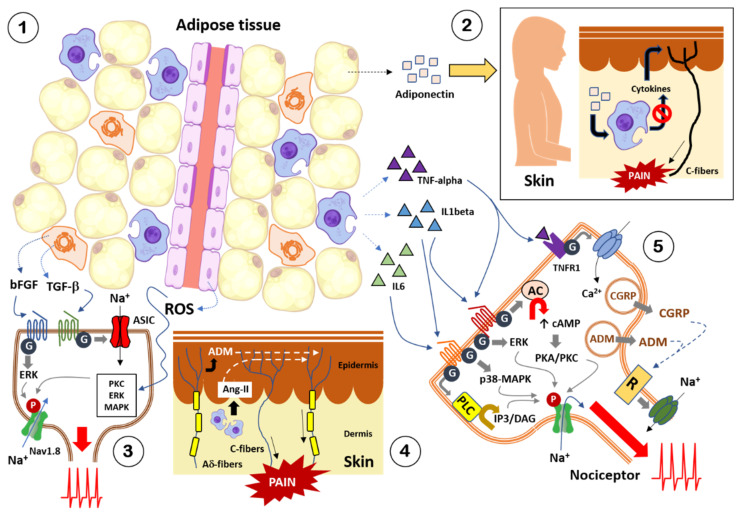
Figure 2.
Crosstalk between adipose tissue, skin, and visceral nociceptors. There is a multitude of diffusible factors secreted by adipose tissue that interact with nociceptors, triggering pain. (1) Adipose tissue is made up of an accumulation of adipocytes, macrophages, lymphocytes, neutrophils, T-cells, natural killer cells, and fibroblasts, as well as an extensive network of blood vessels. (2) Adipocytes secrete adipokines, such as adiponectin, which can reach the skin through the bloodstream. Under an inflammatory condition of the skin, the cutaneous macrophages secrete cytokines, which excite and sensitize the cutaneous nociceptors, triggering pain. Adiponectin acts on these macrophages, inhibiting the release of pain-inducing cytokines. Therefore, this adipokine has analgesic effects. (3) Fibroblasts from adipose tissue accumulated in body tissues and organs secrete growth factors that interact with G protein-coupled receptors on the membrane of nociceptors. Some of these growth factors activate intracellular cascades (e.g., ERK) that allow peripheral sensitization through ion channel phosphorylation (e.g., Nav1.8), thus favoring cation fluxes that trigger depolarization of nociceptors and pain. Other growth factors modulate ion channel opening (e.g., ASIC) and cation entry and depolarization of the nociceptor. Together, growth factors secreted by fibroblasts in adipose tissue cause hyperexcitability of nociceptors and pain. On the other hand, the endothelial cells of the blood vessels that supply adipose tissue secrete oxygen free radicals (ROS) that diffuse to the nociceptors, modulating various intracellular signaling pathways, which cause ion channel phosphorylation, peripheral sensitization and hyperarousal of nociceptors. (4) Inflammation of body tissues, such as the skin, triggers the nociceptors themselves to release adrenomedullin (ADM) or macrophages to release angiotensin-II (Ang-II), two adipokines also secreted by adipose tissue. Both diffusible factors act on receptors in the membrane of cutaneous (or visceral) nociceptors, modulating the opening of ion channels, cation fluxes, and depolarization, causing pain. (5) The macrophages (and other immune cells) of the adipose tissue accumulated in the organs of obese subjects, secrete various cytokines, which by diffusing can interact with specific receptors present in the membrane of visceral nociceptors. The interaction of cytokines is carried out on receptors coupled to G proteins. Some of these interactions (e.g., TNF-alpha) modulate the opening/closing of ion channels, causing variations in the influx of calcium ions. These ions favor the fusion of vesicles loaded with peptides (e.g., pep-tide related to the calcitonin gene or CGRP, adrenomedullin or ADM) present at the terminals of nociceptors. Thus, adipose tissue cytokines cause the release of CGRP and ADM by visceral nociceptors, peptides that interact with their respective receptors (R) that end up regulating the opening of ion channels that favor the entry of sodium ions and depolarization of the nociceptor. At other times, cytokines released by adipose tissue regulate various intracellular cascades (e.g., cAMP/PKA-PKC, ERK, p38-MAPK, IP3/DAG) responsible for the phosphorylation of ion channels and other metabotropic receptors on the nociceptor membrane, triggering peripheral sensitization and hyperexcitability of the nociceptive afferent nerve fiber. In summary, in obese subjects, there is a powerful crosstalk between the accumulated adipose tissue in the body organs and the nociceptive afferent nerve fibers present in these organs, so that the diffusible factors secreted by the adipose tissue cause sensitization and hyperexcitability of the nociceptor, triggering pain. For details, see the main text. Source for figure illustrations: https://scidraw.io/ (accessed on 8 September 2021).