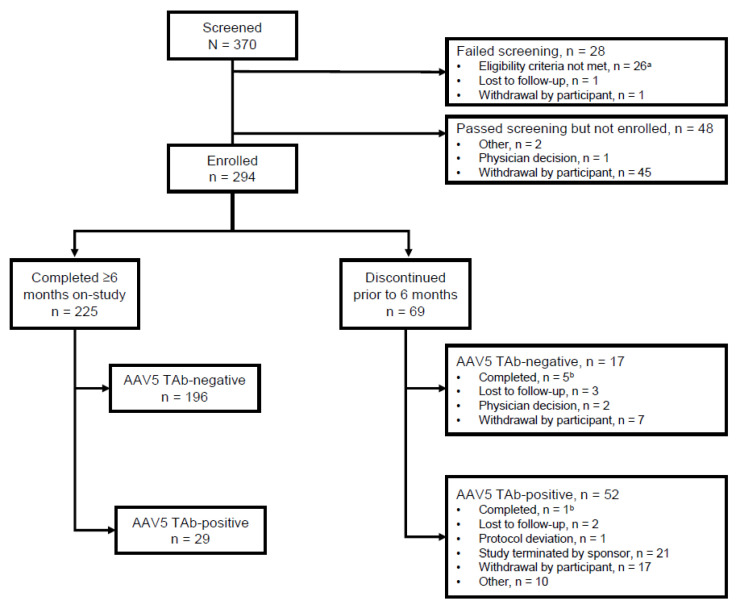
Figure 1.
Patient disposition. a Reasons for screen failure: no history of FVIII inhibitor and results from a Bethesda assay, n = 8; significant liver dysfunction with abnormal laboratory results, n = 3; active hepatitis C, n = 6; chronic or active hepatitis B, n = 1; concurrent enrolment in another clinical study, n = 2; ability to comply with protocol requirements per Investigator, n = 2; male ≥18 years of age with residual FVIII ≤1, n = 1; must have been on prophylaxis FVIII for ≥6 months prior, n = 4. b Completing 6 months on-study was not required for participants not rolling over into the interventional study. AAV5 TAb, adeno-associated virus vector total antibody; FVIII, factor VIII.