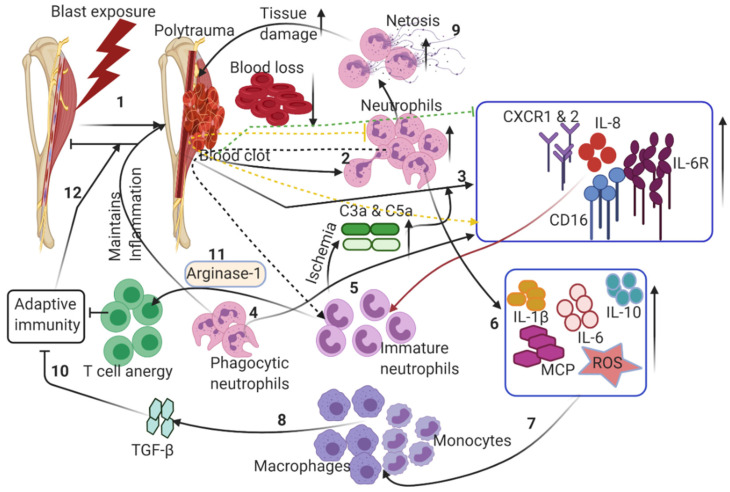
Figure 2.
Dysregulation of innate immune responses following CTI: (1) blast-induced trauma in extremities damages multiple tissues, leading to hematoma. (2) Hematoma triggers neutrophil extravasation from the vasculature to the wound site. (3) A robust increase in pro-inflammatory cytokines and chemokines triggers neutrophils to clear the debris of dead cells. (4) Increased pro-inflammatory chemokines/interleukins induces the release of immature neutrophils, exacerbating the pro-inflammatory conditions. (5) An increase in immature neutrophils induces ischemia through the secretion of chemokines and proteases at the wound site. (6) Increased neutrophil infiltration further causes the upregulation of interleukins, chemokines and reactive oxygen species. (7) Monocytes and macrophages are activated to allow the transition from the acute inflammatory phase to the resolution phase. (8) Activated macrophages induce TGF-β. (9) Netosis of neutrophils induces robust pro-inflammatory responses, leading to excessive tissue damage. (10) Aberrant immune responses restrict the transition to adaptive immune responses. (11) Macrophages secrete arginase 1, thus inducing anergy in T cells. (12) These altered inflammatory responses maintain the presence of triggers for chronic inflammation, thus delaying the wound healing in CTI. Dotted arrows indicate the secondary induction of immune cells/cytokines, which inhibit normal healing responses by exacerbating pro-inflammatory responses. See text for further details.