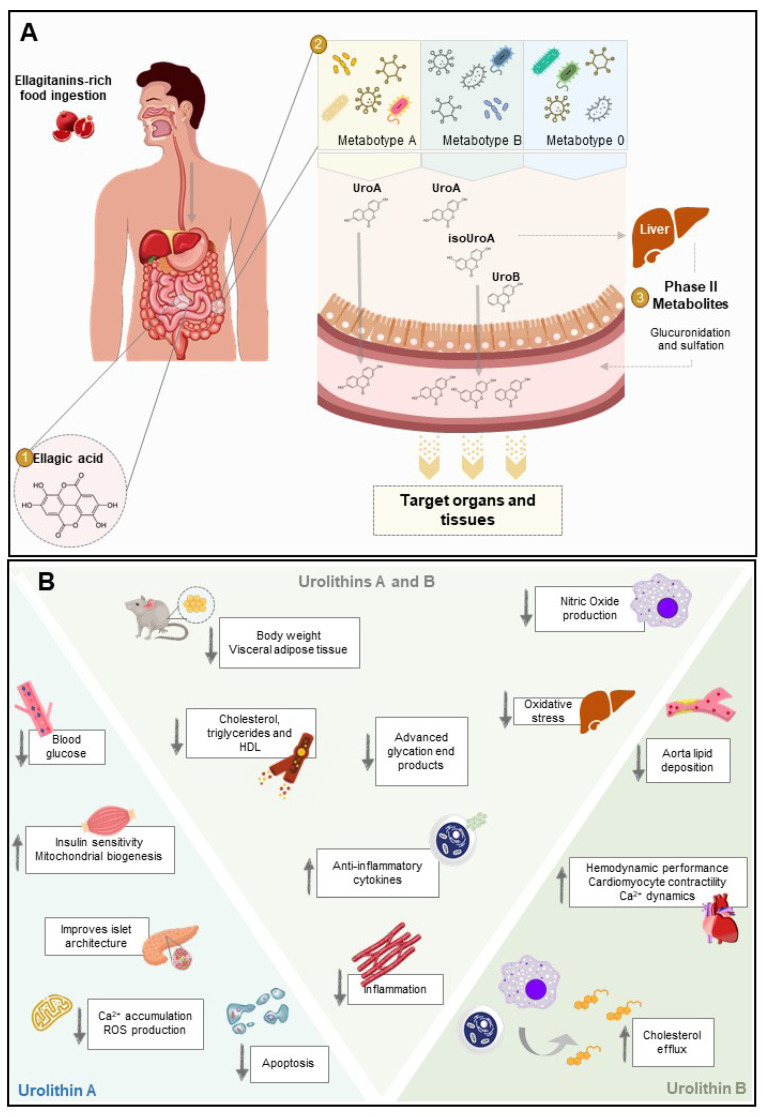
Figure 2.
Metabolism of ellagitannins to yield bioactive urolithins with potential action for diabetes. (A) Several dietary products (e.g., pomegranate) are rich in ellagitannins (ETs), a class of natural (poly)phenols. Upon ingestion, ETs are hydrolyzed in the small intestine into ellagic acid (EA), reaching the colon. Through the action of microbiota, EA is converted to urolithins depending on the individual’s metabotype. In phase-II metabolism, urolithins are further modified by large intestine enterocytes and the liver (methylation, glucuronidation, and sulfation) to yield conjugated forms of these metabolites. Both conjugated and deconjugated urolithins are able to enter circulation and reach the target tissues where they will perform their function. Although conjugated forms are often found at higher concentrations than the respective deconjugated counterparts, aglycones show much higher bioactivity. (B) Once absorbed by the target tissues, urolithin A and urolithin B may exert cell-specific activities that confer protection towards a multitude of diabetes complications. In mice, urolithin A decreases blood glucose and increases glucose tolerance and insulin sensitivity. It also protects against islet architecture disruption, oxidative stress, and cell death. Urolithin B is described to reduce lipid deposition in the aorta and increase cholesterol efflux on macrophages cell lines. In adult Wistar rats with streptozotocin-induced diabetes, urolithin B prevents the negative impact of altered diabetic milieu on cardiac performance. Regarding the common effects, urolithin A and urolithin B influence lipids levels. They reduce body weight and fat mass in high fat diet-fed mice and decrease the amounts of cholesterol, triglycerides, and high-density lipopolysaccharide (HDL) in serum. Urolithin A and urolithin B also protect cells from inflammatory response and oxidative stress.