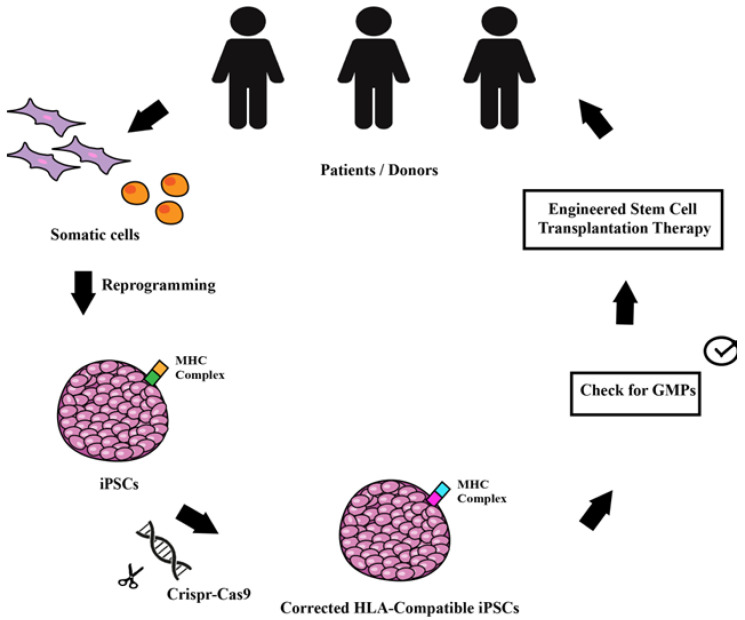
Figure 1.
Schematic drawing of the workflow allowing the use of genetically modified iPSCs for transplantation therapy. Starting from the patients, somatic cells can be reprogrammed into iPSCs that are then corrected or modified with CRISPR/Cas9 gene editing with the aim to modify the MHC complex to make them compatible for a wider population of patients. The engineered stem cells can be used for autologous and/or for allogeneic transplantation and used as therapy for monogenic disorders.