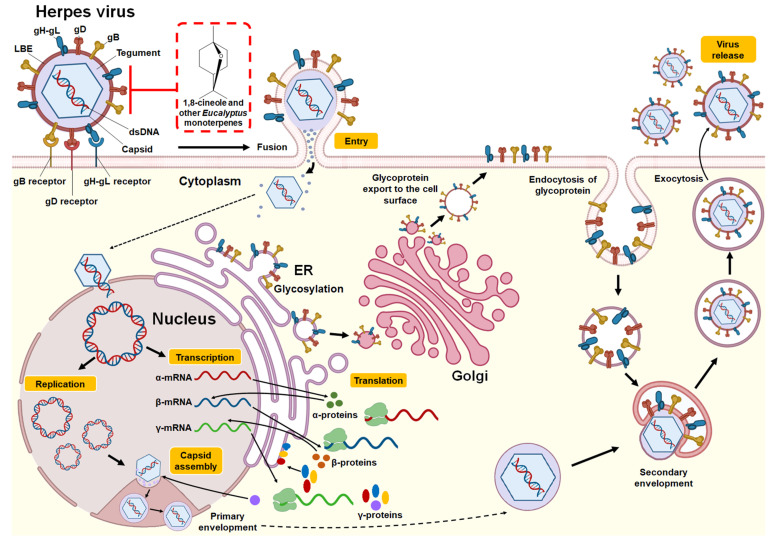
Figure 2.
Structure and replication cycle of Herpes virus. According to Karasneh et al. [46] and Lussignol et al. [47], the Herpes virus consists of 7 structural glycoproteins (gB, gC, gD, gH, gK, gL and gM) present in the lipid bilayer envelope (LBE). However, only four of these glycoproteins (gB, gD, gH, and gL) are necessary and sufficient to allow the fusion of the virus with the plasma membrane of the host cell (shown in the illustration). It has a relatively large, double-stranded, linear DNA genome surrounded by an icosahedral capsid. This, in turn, is surrounded by an integument that contains between 15 and 20 proteins and is in direct contact with the LBE. The herpes virus replication cycle begins when the gB, gD, and gH-gL glycoproteins bind to their receptors in the host cell (gB receptors: PILRα (HSV-1), MAG, NMHC-IIA; gD receptors: HVEM, Nectin-1/Nectin-2, 3-OS HS (HSV-1); gH-gL receptors: αvβ3 integrin). This allows the LBE of the virus to fuse with the plasma membrane or endocytosis, releasing the capsid and integument into the cytoplasm. Using the microtubule network, the nucleocapsid is transported to the nuclear pore, where the viral genome is released into the nucleus and circularized. Viral DNA serves as a template for RNA polymerase II, which leads to the production of mRNA, expressed in three successive and coordinated phases. The mRNAs are translated in the cytoplasm into different viral proteins, including immediate-early (α-proteins), early (β-proteins), and late (γ-proteins) proteins. Most of the late gene products contribute to the formation of the viral particle. Packaging of DNA into preassembled capsids takes place in the nucleus. This is followed by a primary envelope of the capsid by budding through the inner nuclear membrane. The envelope of the perinuclear virions then fuses with the outer nuclear membrane to release naked capsids into the cytoplasm (de-envelopment). The envelope proteins are glycosylated in the endoplasmic reticulum (ER) and then move by transport vesicle from the ER to the Golgi apparatus and finally to the cell plasma membrane. Tegumented capsids acquire a “second” final envelope to become virions from post-Golgi membrane compartments. A role for autophagic membranes in virion envelope and release has been proposed for some herpes viruses. Once formed, virions are transported to the cell surface within small vesicles using exocytosis machinery and released from cells. The red box indicates the potential mechanism of action against the Herpes virus by 1,8-cineole and other monoterpenes present in Eucalyptus essential oils by binding and inhibiting the glycoproteins gB, gD, and gH-gL and thus inhibiting the binding of the virus with its receptors and subsequent fusion of LBE with the host cell.