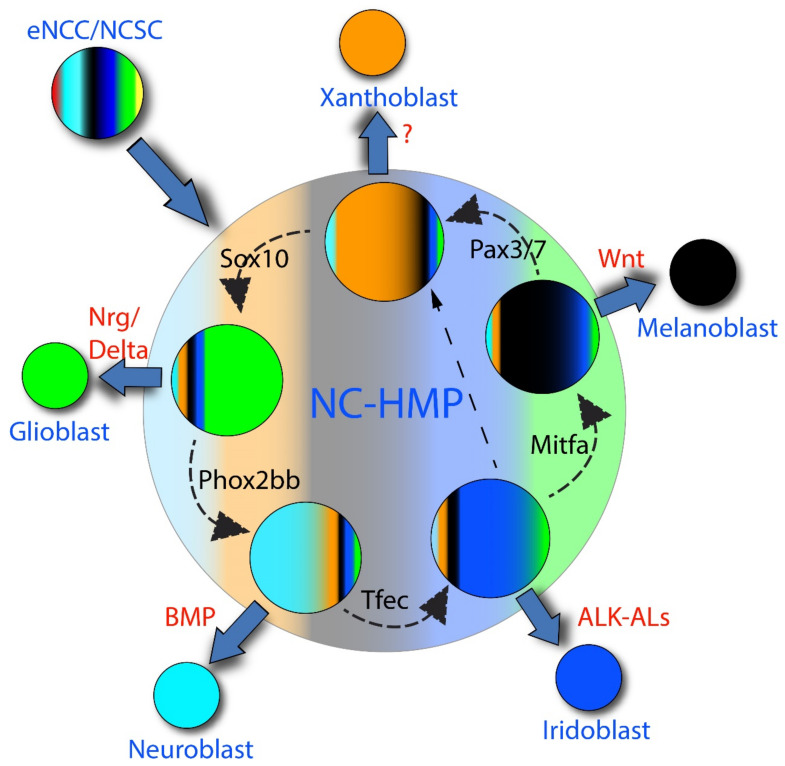
Figure 3.
Putative molecular mechanism underpinning CFR. Multipotent neural crest-derived highly multipotent progenitors (NC-HMP) cycles repeatedly through individual sub-states (circles) biased to be receptive to environmental signals driving specific fate choices (indicated by expanded color on each sub-state); we have proposed that this might simply reflect elevated levels of expression of receptors for fate-specific environmental signals (e.g., Ltk for ALK-ALs involved in iridophore specification; BMPRs for BMPs in sympathetic neuron specification). Transition between sub-states (curved, dashed arrows) is proposed to be dependent upon activity of key fate-specific transcription factors (e.g., Tfec for iridophore; Mitfa for melanocyte; Phox2bb and/or MASH1 for sympathetic neurons). Hence, when these genes are mutated, the NC-HMP lingers in the previous sub-state for a longer period (e.g., in mitfa mutant, cell lingers in pro-iridophore sub-state), although cells eventually overcome this block using a less favored transition (straight, dashed arrow shown for mitfa mutant). Order of transition is predicted based upon mutant phenotypes (e.g., in mitfa mutant, iridophore numbers are elevated), but note that order for other fates is rather more hypothetical and subject to further experimental analysis). Key fate specification environmental factors (red) for melanocyte (Wnt), iridophore (ALK-Als), glia (NRG and Notch ligands, e.g., Delta), sympathetic neurons (BMPs) are deduced from zebrafish or mammalian studies; such signal for xanthophore not quite so clear, but Csf1 is a candidate. Note that in this simplified scheme we have assumed just one type of neuron; of course, other neuron types (e.g., sensory neurons) are derived from the NC-HMP.