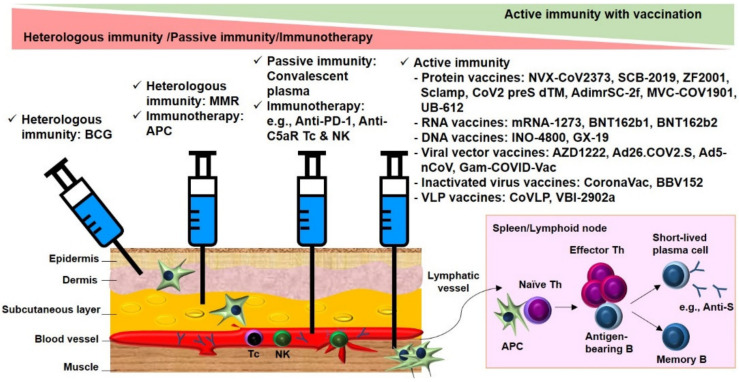
Figure 4.
Summary of clinical trials on immune augmentation against SARS-CoV-2 infection. Clinical trials ranged from off-the-shelf BCG or MMR vaccines that aimed at inducing protective heterologous immunity against COVID-19 for healthcare professionals, to direct transfer of hyperimmunoglobulin or ex vivo trained immune cells that aimed at preventing viral dissemination or direct killing of infected cells in COVID-19 patients, then to conventional vaccines with protein vaccines, RNA vaccines, DNA vaccines, viral vector vaccines, inactivated virus vaccines, and VLP vaccines that aimed at COVID-19 prophylaxis via eliciting Th-dependent B memory pathways in healthy adults. BCG, Bacillus Calmette–Guérin; APC, antigen-presenting cell; PD-1: programmed cell death protein-1; Tc, cytotoxic T; NK, natural killer; C5aR, component 5a receptor; VLP, virus-like particle.