FIGURE 2.
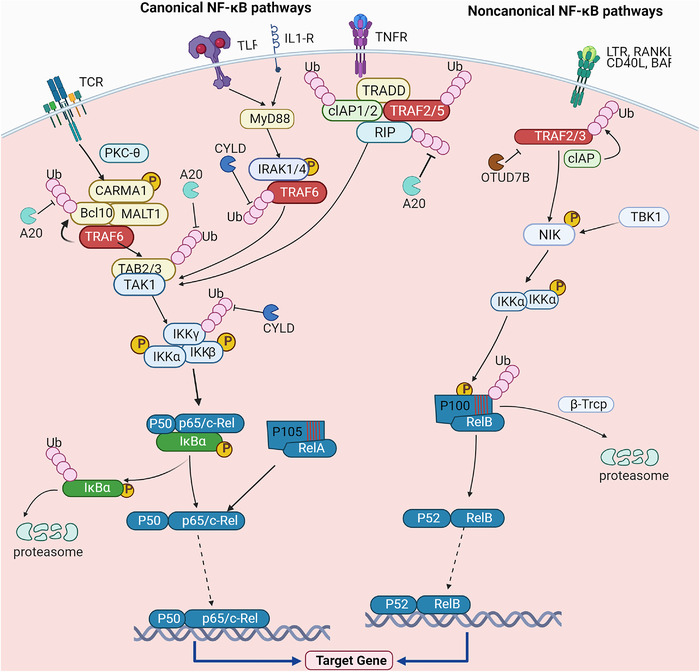
Activation of canonical and noncanonical NF‐κB pathways. In the canonical NF‐κB pathway, protein kinase C‐θ recruits Bcl10 and membrane‐associated lymphoid tissue 1 (MALT1) to form a CARD‐containing MAGUK protein 1 (CARMA1)/Bcl10/membrane‐associated lymphoid tissue (MALT) 1 complex after T‐cell receptor stimulation. TRAF6 is recruited by MALT1, which mediates the ubiquitination of itself and Bcl10 and further activates TAK1. TAK1 phosphorylates and activates IKKβ to induce phosphorylation and degradation of IκBα. After IκBα is degraded, free NF‐κB enter the nucleus to drive target gene transcription. TRADD, the interacting E3 ubiquitin ligases cellular inhibitor of apoptosis (cIAP) 1/2, TRAF2/5, and protein kinase RIP are recruited by tumor necrosis factor receptor (TNFR) stimulation. RIP is then ubiquitinated and recruited to form the TAK1‐IKK complex, which induces NF‐κB activation. Under the stimulation of Toll‐like receptor and interleukin‐1 (IL‐1), myeloid differentiation primary response 88 (MyD88)‐dependent signal transduction induces the recruitment of IRAK1/4 and TRAF6 to activate TAK1‐IKK complex, which then activates NF‐κB. In the noncanonical NF‐κB pathway, specific subsets of the TNFR superfamily are engaged with their ligands to induce the recruitment of TRAF2/3‐cIAP. After TRAF3 is ubiquitinated and degraded, NF‐κB‐induced kinase accumulates in the cytoplasm and then binds to IKKα, mediating p100 phosphorylation and ubiquitination‐dependent processing to produce p52/RelB heterodimers. In these processes, A20, cylindromatosis, and Otud7b play important roles in inhibiting ubiquitination of key molecules of NF‐κB pathways
