Abstract
Simple Summary
Spodoptera cilium Guenee (Lepidoptera: Noctuidae) is one of the grass pests in some parts of the world, including the southern regions of Iran. The larvae of S. cilium feed on grasses and heavy infestations can severely destroy lawn grasses. In the present study, we monitored the effects of Imunit on some biological and demographic parameters of the offspring generation. Our results indicate that Imunit reduced the survival rate and fecundity of S. cilium and could be used in the management programs of this pest.
Abstract
Imunit is a mixture of alpha-cypermethrin + teflubenzuron, and has been launched for controlling caterpillars. In this study, the effects of Imunit at LC50 and LC30 were investigated on parental and offspring generation of S. cilium, according to age-stage, two-sex life table. The experiments were conducted by leaf dipping method at 25 °C and 60 ± 5% relative humidity, under a cycle of 16 h fluorescent light and 8 h darkness. LC30 and LC50 concentrations of Imunit increased the immature developmental time of S. cilium in the offspring generation, while the LC50 of Imunit significantly reduced the developmental time of adults. The adult pre-oviposition period and total pre-oviposition period considerably increased when offspring were treated with LC50 of Imunit. In offspring of S. cilium exposed to LC50 and LC30 concentrations of Imunit, the gross reproductive rate (GRR), net reproduction rate (R0), the intrinsic rate of population increase (r), and the finite rate of population increase (λ) significantly reduced compared to the control. This study showed that the application of Imunit at LC50 could suppress the S. cilium population and can be used in the integrated management program of this pest.
Keywords: grass, insecticide, life table, Spodoptera cilium
1. Introduction
The genus Spodoptera moths migrate in clusters and feed on numerous plants. Due to the wide range of hosting and global distribution, Spodoptera species are important pests of crops. Adults of Spodoptera are active at night and classify as a nocturnal pest, but their larvae are active and feeding on plants during the day [1]. The grass-lawn armyworm Spodoptera cilium Guerine (Lepidoptera: Noctuidae) is considered a major pest of Graminaceous plants [2], and classified as the main limitation of rice yield in African countries [3].
The indiscriminate use of insecticides, including chemical and bio-insecticides, especially those with a wide range, has led to ecological imbalances, environmental hazards, severe disruptions to natural and agricultural ecosystems. Additionally, increasing insecticide pressure leads to pest resistance to many typical insecticides, resulting in pests’ re-emergence [4,5]. Sub-lethal concentrations affects the biological characteristics, physiology, behavior, and parameters of the life table of insects. For example, sub-lethal concentrations affect the population growth of insects and their life table parameters [6]. Several biological effects of sub-lethal concentrations of insecticides have been reported in the literature [7,8,9]. Sub-lethal effects of the pesticides including lufenuron, methoxyfenozide, spinosad, endosulfan, novaluron, and tebufenozide resulted in the reduction in pupal weight, adult developmental period time, and fertility of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) [7]. The demographic parameters of Plutella xylostella L. (Lepidoptera: Plutellidae), in several generations, was affected by the LC25 concentration of the insecticide spinosad [8]. The insecticide hexaflumuron reduced the total number of eggs laid by each female, oviposition period, and adult emergence time of P. xylostella [10]. Reproductive life tables for LC10, and LC25 concentrations of the insecticide chlorantraniliprole on diamondback moth illustrated that the developmental period of the larvae and pupae increase after using this pesticide [11]. The sub-lethal effects of spinosad can affect the population dynamics of Spodoptera exigua Hübner (Lepidoptera: Noctuidae) by reducing the survival rate, reproduction, and development time of this species [9]. Effects of cyantraniliprole at LC30 reduced the pupal weight and fertility of Helicoverpa assulta Guenée (Lepidoptere: Noctuidae). However, this insecticide did not significantly affect the development period of pupae and adult emergence [12]. The effectiveness of five insecticides was evaluated to control S. cilium under laboratory and field conditions in the local area of lawns. Spodoptera cilium created brown patches and bare spots in the lawn. However, among the tested insecticides, Imunit was the most active against grass-lawn armyworms and reduced the damage percentage (28%) of this pest [13].
Imunit 150 SC is a mixture of alpha-cypermethrin (7.5%) and teflubenzuron (7.5%). According to data from the Insecticide Resistance Action Committee (IRAC) classification, alpha-cypermethrin belongs to the sodium channel modulating neurotoxins (group 3A) and the subtype of pyrethroids. Teflubenzuron is in the IRAC classification member of group 15, which be included in the class of insect growth regulators and inhibitors of chitin biosynthesis and the benzene-urea subgroup [14].
The purpose of the current study was to evaluate the effect of LC50 and LC30 concentrations of Imunit on S. cilium through investigating the biological characteristics and life table parameters of the pest.
2. Materials and Methods
2.1. Rearing of Spodoptera Cilium
The larvae of S. cilium were collected from infested green-lawn spaces in Gachsaran, Iran, in July 2019. Collected larvae were fed on grass leaves until the emergence of adult insects and identified based on morphological characters. A mixture of 50% Bermudagrass (Cynodon dactylon (L.) Pers.), and 50% Sport grass (including 80% Lolium perenne L., 10% Festuca rubra cv. Reverent, 10% Poa pratensis L.) seeds were planted in the greenspace in Dogonbadan Municipality, Gachsaran, and used for rearing the grass-lawn armyworms. Collected larvae were introduced to cylindrical plastic containers (15 cm in diameter and 15 cm in height) and grass leaves were provided to the larvae daily. The lids of the containers were enclosed with piece of muslin for ventilation. Each container was set down in the growth chamber regulated at 25 ± 1 °C, 60 ± 5% relative humidity (RH), and a cycle of 16 h fluorescent light and 8 h dark. Adult moths were fed with a 20% honey solution impregnated onto cotton wool. Field collected insects were reared for three generations to acclimate the insects to laboratory conditions. Third-instars from the fourth generation were used in the experiments.
2.2. Insecticide Tested
Imunit (150 SC) is a mixture of alpha-cypermethrin (7.5%) and teflubenzuron (7.5%), and was supplied by BASF, Ludwigshafen, Germany.
2.3. Dose-Mortality Response Bioassay
Bioassays were conducted using the leaf dip method. Grass leaves (two grams/dish) were dipped in different concentrations (25, 50, 90, 150, and 180 ppm) of the Imunit insecticide solution for 10 s. Distilled water was served as a control. The treated leaves were air-dried for one hour at room temperature and placed in plastic Petri dishes (12 cm in diameter) with a hole (2 cm in diameter) capped with a muslin cloth. In each Petri dish, 25 third-instar larvae were released, and the experiment was replicated three times. Percentage larval mortality was counted 48 h after treatment using a Nikon SMZ800 stereomicroscope (Japan). All experiments were performed in the growth chamber set at 25 ± 1 °C, 60 ± 5% RH, and 16:8 h (L:D) photoperiod.
2.4. Imunit Effects on Parental Generation
Grass leaves (two grams/dish) were dipped in LC30 (18.2 ppm) and LC50 (27.7 ppm) concentrations of Imunit insecticide solution for 10 s. Distilled water served as a control. The treated leaves were air-dried for one hour at room temperature and placed in plastic Petri dishes (12 cm in diameter) with a hole (2 cm in diameter) capped with a muslin cloth. Then, 60 third-instar larvae were released separately into each Petri dish and kept under experimental conditions for 48 h. After 48 h, the surviving larvae were separately transferred to untreated leaves in plastic Petri dishes. The Petri dishes were checked daily until pupation, and developmental time was recorded for all immature stages sing a Nikon SMZ800 stereomicroscope (Japan). After the moth imago emergence, females were coupled with males obtained from the selfsame treatment in separate plastic containers (11 cm length × 9 cm width × 4 cm height) containing 20% honey solution as food. The adults were transferred to new containers every day, and the total number of eggs laid was counted daily. This procedure was continued until all the adults died. All experiments were performed in the growth chamber set at 25 ± 1 °C, 60 ± 5% RH, and 16:8 h (L:D) photoperiod.
2.5. Imunit Effects on Offspring Generation
To assess the effects of Imunit to the offspring generation, 100 eggs produced from parents were transmitted separately to the test Petri dishes containing 2 g grass leaves. Fresh leaves (two grams/dish) were added daily to the dishes, and the immature development time was recorded every day. As mentioned above, when adults emerged, female moths were coupled with males, and if the male died before the female, a new male was transferred to the container.
2.6. Data Analysis
Data were subjected to Probit analysis (Finney 1971) to estimate LC30 and LC50 concentrations of Imunit using SAS 6.12 software (SAS Institute 1997). The development time of the third- to fifth-instar larvae, pupae, adult, and oviposition period of the parental generation and also, the development time of the offspring generation including duration of eggs, first- to fifth-instar larvae, pupae, pre-adult, adult, oviposition period, adult pre-ovipositional period (APOP), and total pre-ovipositional period (TPOP) were analyzed using the age–stage, two-sex life table [15,16] using TWOSEX MS Chart software [17]. From the output of this software we extracted the following parameters: Age-specific survival rate (lx), Age-specific fecundity of total population (mx), Gross reproductive rate (GRR), net rate of reproduction (R0), intrinsic rate of population growth (r), finite rate of population growth (λ), and the average period length of one generation (T). The intrinsic rate of population growth was obtained from the Euler–Lotka formula, with age indexed from zero, according to Goodman [18]. Age–stage-specific life expectancy (exj) (x: insect age (days) and j: insect growth stage (period)) was also calculated. The mean and standard error of life table indices were estimated using the Bootstrap method [19]. The paired Bootstrap test based on confidence limits was used to compare the means.
3. Results
3.1. Estimation of Concentrations
The LC30 and LC50 concentrations of Imunit on third instar larvae were 18.2, and 27.7 ppm, respectively (Table 1).
Table 1.
Toxicity of Imunit on third-instar larvae of Spodoptera cilium.
| Insecticide | Number of Larvae Tested |
df | LC50 (ppm) | LC30 (ppm) | Chi Square | p Value |
|---|---|---|---|---|---|---|
| Imunit | 375 | 3 | 27.7 | 18.2 | 3.60 | 0.40 |
3.2. Imunit Effects on Parental Generation
The results of parental generation (F0) treatment indicated that there were no significant differences in developmental time of third- and fifth-instar larvae of S. cilium after being treated with LC30 and LC50 of Imunit in comparison with the control group. In contrast, the development time of pupae was significantly longer in the treatment group compared to the control group. However, the time of adult emergence and oviposition period were significantly increased in the LC30 and LC50-treated group of Imunit compared to the control (Table 2).
Table 2.
Effects of Imunit on developmental time, and fecundity of female adult (Mean ± SE) of Spodoptera cilium parental generation (F0).
| Life Stage | Treatments | ||
|---|---|---|---|
| Control | LC30 | LC50 | |
| Third-instar larvae (d) | 4.00 ± 0.041 a | 4.10 ± 0.03 a | 4.08 ± 0.3 a |
| Fourth-instar larvae (d) | 3.93 ± 0.042 b | 4.06 ± 0.03 a | 4.03 ± 0.02 a |
| Fifth-instar larvae (d) | 4.15 ± 0.08 a | 4.07 ± 0.05 a | 4.05 ± 0.05 a |
| Pupae (d) | 5.23 ± 0.06 c | 6.48 ± 0.17 b | 7.17 ± 0.19 a |
| Adult (d) | 8.28 ± 0.17 a | 7.52 ± 0.31 a | 5.61 ± 0.43 b |
| Oviposition (d) | 4.83 ± 0.23 a | 3.93 ± 0.25 b | 3.40 ± 0.45 b |
| Fecundity (eggs/female) | 837.4 ± 22.54 a | 514.3 ± 43.89 b | 434.3 ± 24.95 c |
Standard errors were estimated by using the bootstrap technique with 100,000 resampling. Means followed by the same letter within a row are not significantly different using the paired bootstrap test based on the confidence interval of difference (p < 0.05).
3.3. Imunit Effects on Offspring Generation
For offspring generation (F1), the pre-adult development time was significantly prolonged on the LC30 (34.56 d) and LC50 treatments (37.67 d) of Imunit in comparison to the control (29.63 d). However, the adult and oviposition periods were significantly shorter on the LC30- and LC50-treated groups of Imunit. This insecticide significantly extended the APOP, TPOP, and female longevity at LC30 and LC50 concentrations compared with the control. However, the male longevity of S. cilium was not affected by the LC30 concentration of Imunit compared to the control group (Table 3).
Table 3.
Effects of Imunit on developmental time (Mean ± SE) of Spodoptera cilium offspring generation (F1).
| Life Stage | Treatments | ||
|---|---|---|---|
| Control | LC30 | LC50 | |
| Egg (d) | 4.03 ± 0.04 c | 4.20 ± 0.04 b | 4.78 ± 0.04 a |
| First-instar larvae (d) | 4.02 ± 0.01 c | 4.28 ± 0.04 b | 4.44 ± 0.05 a |
| Second-instar larvae (d) | 4.09 ± 0.03 b | 4.22 ± 0.04 b | 4.62 ± 0.05 a |
| Third-instar larvae (d) | 4.03 ± 0.03 a | 4.08 ± 0.03 a | 4.12 ± 0.05 a |
| Fourth-instar larvae (d) | 4.07 ± 0.04 c | 4.42 ± 0.06 b | 4.96 ± 0.26 a |
| Fifth-instar larvae (d) | 4.16 ± 0.04 b | 4.55 ± 0.27 b | 5.23 ± 0.10 a |
| Pupae (d) | 5.33 ± 0.05 b | 9.88 ± 0.12 a | 9.50 ± 0.34 a |
| Pre-adult (d) | 29.63 ± 0.09 c | 34.56 ± 0.18 b | 37.67 ± 0.33 a |
| Adult (d) | 7.91 ± 0.08 a | 3.81 ± 0.53 b | 3.83 ± 0.91 b |
| Oviposition (d) | 5.61 ± 0.17 a | 2.00 ± 0.00 b | 1.00 ± 0.00 c |
| APOP 1 (d) | 1.14 ± 0.07 c | 2.67 ± 0.33 b | 3.33 ± 0.33 a |
| TPOP 2 (d) | 30.61 ± 0.16 c | 37.33 ± 0.33 b | 41.00 ± 0.00 a |
| Fecundity (eggs/female) | 728.21 ± 85.70 a | 306.33 ± 82.28 b | 195.0 ±55.0 c |
| Adult longevity (male) (d) | 37.52 ± 0.17 b | 37.77 ± 0.68 b | 40.75 ± 1.38 a |
| Adult longevity (female) (d) | 37.57 ± 0.21 c | 41.00 ± 0.58 b | 43.00 ± 0.57 a |
| Total longevity (all individuals) (d) | 35.43 ± 0.407 a | 28.18 ± 0.690 b | 27.09 ± 0.88 b |
Standard errors were estimated by using the bootstrap technique with 100,000 resampling. Means followed by the same letter within a row are not significantly different using the paired bootstrap test based on the confidence interval of difference (p < 0.05). 1 Adult pre-oviposition period, time between adult emergence and first oviposition. 2 Total pre-oviposition period, time from birth to first reproduction in female.
The results demonstrated that the LC30 and LC50 of Imunit significantly reduced gross reproductive rate (GRR), net reproductive rate (R0), intrinsic rate of population growth (r), and finite rate of population increase (λ) compared to the control. The mean generation time of offspring generation (F1) showed a significant increase in the LC30- (38.78 d) and LC50-treated (42.0 d) group of Imunit compared to the control (33.87 d) (Table 4).
Table 4.
Effects of Imunit on the population parameters (Mean ± SE) of Spodoptera cilium offspring generation (F1).
| Parameters | Treatments | ||
|---|---|---|---|
| Control | LC30 | LC50 | |
| GRR (offspring/female) | 282.9 ± 52.75 a | 114.9 ± 55.65 b | 97.5 ± 51.41 b |
| R0 (offspring/female) | 203.9 ± 40.15 a | 9.190 ± 5.32 b | 3.900 ± 2.58 b |
| r (d−1) | 0.160 ± 0.01 a | 0.050 ± 0.02 b | 0.032 ± 0.01 c |
| λ (d−1) | 1.170 ± 0.01 a | 1.050 ± 0.02 b | 1.030 ± 0.01 b |
| T (d) | 33.87 ± 0.27 c | 38.78 ± 0.26 b | 42.00 ± 0.02 a |
Standard errors were estimated by using the bootstrap technique with 100,000 resampling. Means followed by the same letter within a row are not significantly different using the paired bootstrap test based on the confidence interval of difference (p < 0.05).
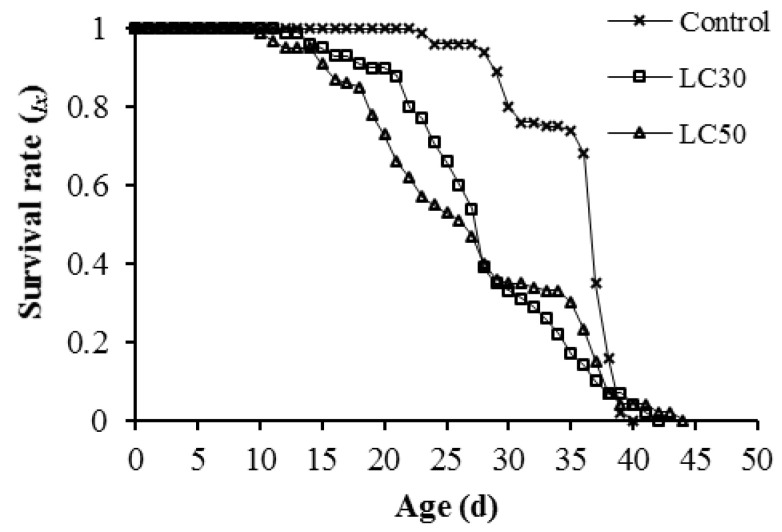
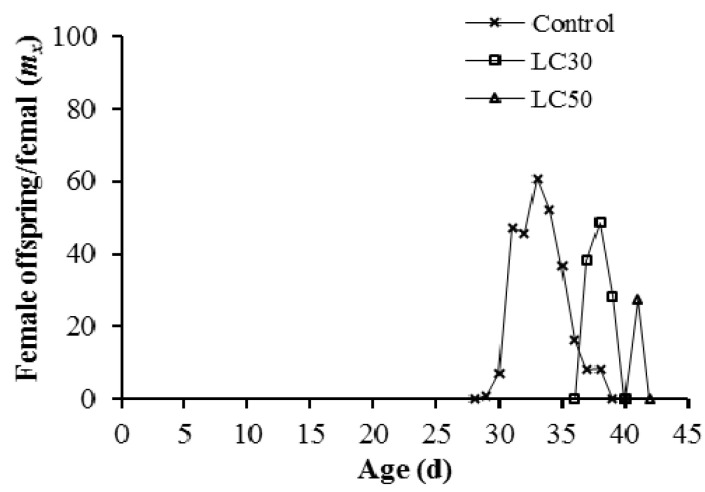
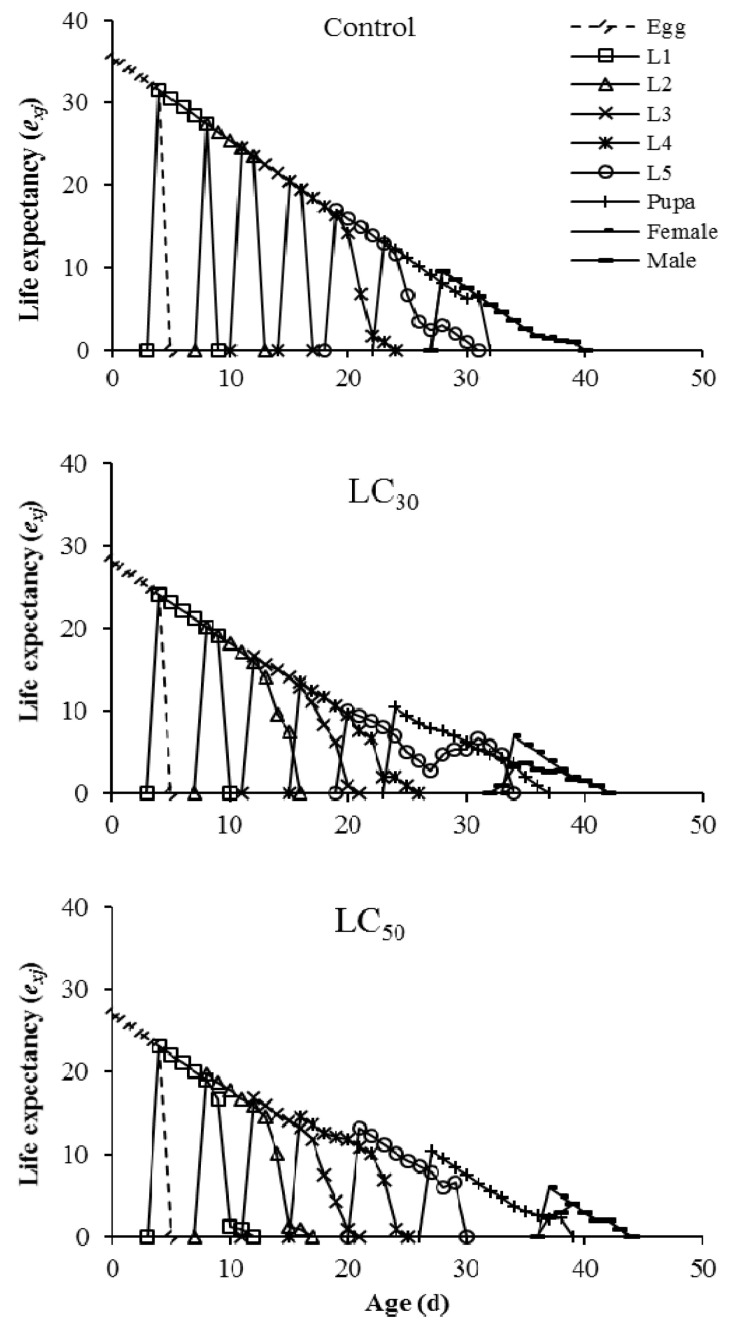
The age-specific survival rate (lx) indicated a slight decrease within the first 18 and 21 d in LC30 and LC50 treatments, respectively (Figure 1). The fecundity level in the LC30 and LC50 treatments was lower than in the control. The highest mean number of hatched eggs produced by each individual was reported on day 33 (60.72 eggs) in the control group (Figure 2). The number of days that an individual of age x and stage j is expected to survive is shown by the age-specific life expectancy curve (exj). In offspring generation (F1), the exj in the control treatment was more than theLC30 and LC50-treated groups (Figure 3).
Figure 1.
Age-specific survival rate (lx) of Spodoptera cilium of offspring generation (F1) exposed to LC30 and LC50 concentrations of Imunit.
Figure 2.
Age-specific fecundity of total population (mx) produced by Spodoptera cilium of offspring generation (F1) exposed to LC30 and LC50 concentrations of Imunit.
Figure 3.
Age–stage specific life expectancy (exj) of Spodoptera cilium offspring generation (F1) exposed to LC30 and LC50 concentrations of Imunit.
4. Discussion
Limiting the number and concentration of pesticides used is one of the important goals of integrated pest management (IPM), reducing the risks to human health, the environment, and beneficial insects [20]. It is necessary to find an insecticide that can sufficiently reduce the population and damage of grass-lawn armyworm S. cilium. In our previous study, Imunit had the highest insecticidal activity on larvae of S. cilium compared to other tested insecticides, including indoxacarb, fenvalerate, diflubenzuron, and a commercial formulation of the bacterium Bacillus thuringiensis [13]. Accordingly, Imunit was selected from the typical insecticides used against S. cilium to conduct an investigation into the sub-lethal effects on biological characteristics and life table parameters of this pest.
Our results showed that Imunit used in LC30 concentration significantly extended the developmental time of S. cilium pre-adult stages but decreased the adults’ duration in the offspring generation. One of the active ingredients of Imunit is teflubenzuron, a chitin biosynthesis inhibitor in the benzoylurea class that interferes with the molting of insects [21]. The inhibitory activity of teflubenzuron on the formation of chitin has been reported in Spodoptera littoralis Boisduval [22]. Very little has been known about the sublethal effects of teflubenzuron on the population and demographic parameters of different species of the genus Spodoptera (Lepidoptera: Noctuidae). Methoxyfenozide is an ecdysone receptor agonist (diacylhydrazine class), and at LC25 significantly increased larval and pupal growth time of S. exigua. The increase of larval development time may be due to the induction of additional molting in larvae, which arises from the use of ecdysone agonists [23]. Hexaflumuron (benzoylurea class) at LC10 and LC25 concentrations prolonged the development duration of pre-adult stages, rather than shortening the adults’ longevity. Moreover, hexaflumuron at sublethal concentrations decreased the fecundity of P. xylostella [24]. However, diflubenzuron, another insecticide of the benzoylurea class, did not affect the growth and reproduction of Platynota idaeusalis Walker (Lepidoptera: Tortricidae), but it increased the mortality of male pupae. Diflubenzuron was reported as the weakest insecticide in its class [25]. Lufenuron, another benzoylurea insecticide, at LC50 decreased the size and weight of P. xylostella pupae, but the larval and pupal growth period was not affected. Moreover, lufenuron significantly reduced the adult emergence [26]. Teflubenzuron inhibited larval molting, larval-pupal ecdysis and adult emergence of Leptinotarsa decemlineata (Say). Teflubenzuron was noted to have antifeedant activity and reduced larval foliage-feeding, which leads to a reduction in the consumption rate of the larvae and their weakening. Moreover, teflubenzuron ingestion delayed the development of L. decemlineata larvae resulted in prolongation of larval period [27]. The larvae of Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae) exposed to the insecticide, cyantraniliprole, expend more energy on detoxification than on growth, thus increasing their developmental duration compared to the control [28].
In our study, the oviposition period and fecundity of both parent and offspring generations were significantly decreased at LC30 and LC50 concentrations of Imunit. Azadirachtin reduced fecundity in African armyworm, Spodoptera exempta Walker at doses of 0.01 and 0.1 µg of the active ingredient per larva as a topical application [29]. Similar effect was observed in S. littoralis when this insecticide was added to the diet of larvae at 0.001 ppm concentration [30]. Azadirachtin and methoxyfenozide (ecdysone agonist) at 100 mg (a.i) per liter influenced the population dynamics of S. littoralis and significantly reduced adult longevity, fecundity, and fertility [31]. Teflubenzuron, like other chitin synthesis inhibitors, adversely affects the fecundity of moths [25,26].
The life table parameters GRR, R0, r, and λ decreased significantly in the progeny generation of S. cilium compared to the control group. Similar outcomes were reported by [9], when spinosad was applied in food of S. exigua at a sublethal concentration of 0.30 mg (a.i) per kg diet weight. This concentration significantly reduced the R0 and r values in comparison to the control group. Similarly, demographic growth parameters (R0, r, and λ) of P. xylostella offspring generation were significantly reduced when treated with LC25 of chlorantraniliprole in comparison with the control [32]. Our results also illustrated that Imunit, even at sublethal concentration, had an adverse influence on other parameters such as lx, mx, and exj, indicating Imunit inhibited the survival rate and life expectancy of S. cilium.
Another active ingredient in this insecticide is alpha-cypermethrin. Cypermethrin at LC10, LC30, and LC50 concentrations significantly decreased adult longevity, fecundity, fertility, as well as adult emergence of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Furthermore, demographic growth parameters of the chickpea pod borer, H. armigera, including R0, r, and λ are negatively affected by cypermethrin [33]. Beta-cypermethrin at LC15, LC30, and LC50 values significantly prolonged the developmental duration of P. xylostella larvae than the control group. The oviposition period of offspring generation was shortened, and a significant fecundity reduction was detected in LC10, and LC30, treated groups. Moreover, mean values of r, λ, GRR, and R0 were significantly lower in all the treated groups than in the untreated control group [34]. These results are in accordance with our findings. Imunit negatively affected population growth and demographic parameters of S. cilium. Quan et al. [35] reported that beta-cypermethrin at LC10 significantly reduced fertility and adult longevity of Carposina sasakii Matsumura (Lepidoptera: Carposinidae). The age-specific survival rate (lx) of the adults was also adversely affected by the LC10 concentration of beta-cypermethrin, but this insecticide increased the duration of the oviposition period and stimulated fecundity [35].
5. Conclusions
The present study suggests that both active ingredients in Imunit (alpha-cypermethrin + teflubenzuron) can effectively reduce the grass-lawn armyworm, S. cilium population. Imunit suppress the population growth of S. cilium. Imunit, at low concentrations, can be considered in integration with other low-risks methods for controlling S. cilium in green spaces.
Acknowledgments
The authors are appreciated from Shahid Chamran University (Grant No. 14909) and also thanks to Dogonbadan Municipality for logistic support of this project. Special thanks to Chi for his constructive comments on the results.
Author Contributions
M.H. and M.Z.; Conceptualization, methodology, investigation, formal analysis, validation, writing—original draft preparation, M.Z.; supervision, visualization, A.A.S.; supervision, writing—review and editing, M.M.-K.; writing—review and editing, and J.F.; visualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Shahid Chamran University of Ahvaz (grant No. SCU. 14909).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the M.Z.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Capinera J. Order Lepidoptera—Caterpillars, Moths and Butterflies. In: Capinera J., editor. Handbook of Vegetable Pests. Academic Press; San Diego, CA, USA: 2020. pp. 353–510. [Google Scholar]
- 2.Brown E., Dewhurst C. The genus Spodoptera (Lepidoptera, Noctuidae) in Africa and the near east. Bull. Entomol. Res. 1975;65:221–262. doi: 10.1017/S0007485300005939. [DOI] [Google Scholar]
- 3.Heinrichs E.A., Barrion A.T. Rice-Feeding Insects and Selected Natural Enemies in West Africa: Biology, Ecology, Identification. International Rice Research Institute and Abidjan (Côte d’Ivoire), WARDA—The Africa Rice Center; Los Baños, Philippines: 2004. [Google Scholar]
- 4.Aktar M.W., Sengupta D., Chowdhury A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009;2:1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegwart M., Graillot B., Blachere Lopez C., Besse S., Bardin M., Nicot P.C., Lopez-Ferber M. Resistance to bio-insecticides or how to enhance their sustainability: A review. Front. Plant Sci. 2015;6:381. doi: 10.3389/fpls.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De França S.M., Breda M.O., Barbosa D.R., Araujo A.M., Guedes C.A. The sublethal effects of insecticides in insects. In: Shields V.D.C., editor. Biological Control of Pest and Vector Insects. IntechOpen; London, UK: 2017. pp. 23–39. [Google Scholar]
- 7.Storch G., Loak A.E., Borba R.S., Magano D.A., Moraes C.L., Grutzmacher C.L. The effect of sub-lethal doses of insecticides on artificial diet and caterpillars of Anticarsia gemmatalis (Lepidoptera: Noctuidae) Rev. Bras. Agrociência. 2007;13:175–179. [Google Scholar]
- 8.Yin X.-H., Wu Q.-J., Li X.-F., Zhang Y.-J., Xu B.-Y. Demographic changes in multigeneration Plutella xylostella (Lepidoptera: Plutellidae) after exposure to sublethal concentrations of spinosad. J. Econ. Entomol. 2009;102:357–365. doi: 10.1603/029.102.0146. [DOI] [PubMed] [Google Scholar]
- 9.Wang D., Wang Y.-M., Liu H.-Y., Xin Z., Xue M. Lethal and sublethal effects of spinosad on Spodoptera exigua (Lepidoptera: Noctuidae) J. Econ. Entomol. 2013;106:1825–1831. doi: 10.1603/EC12220. [DOI] [PubMed] [Google Scholar]
- 10.Mahmoudvand M., Abbasipour H., Garjan A.S., Bandani A.R. Decrease in pupation and adult emergence of Plutella xylostella (L.) treated with hexaflumuron. Chil. J. Agric. Res. 2012;72:206–211. doi: 10.4067/S0718-58392012000200007. [DOI] [Google Scholar]
- 11.Han W., Zhang S., Shen F., Liu M., Ren C., Gao X. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae) Pest Manag. Sci. 2012;68:1184–1190. doi: 10.1002/ps.3282. [DOI] [PubMed] [Google Scholar]
- 12.Dong J., Wang K., Li Y., Wang S. Lethal and sublethal effects of cyantraniliprole on Helicoverpa assulta (Lepidoptera: Noctuidae) Pest Biochem. Physiol. 2017;136:58–63. doi: 10.1016/j.pestbp.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Hatami M., Seraj A., Ziaee M., Mehrabi-Koushki M. Efficacy of different insecticides in control of Spodoptera cilium in laboratory and field conditions. Plant Pests Res. 2020;10:1–15. [Google Scholar]
- 14.Oberlander H., Silhacek D. Insecticides with Novel Modes of Action. Springer; Berlin, Germany: 1998. New perspectives on the mode of action of benzoylphenyl urea insecticides; pp. 92–105. [Google Scholar]
- 15.Chi H., Liu H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985;24:225–240. [Google Scholar]
- 16.Chi H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988;17:26–34. doi: 10.1093/ee/17.1.26. [DOI] [Google Scholar]
- 17.Chi H. TIMING-MSChart: Computer Program for Population Projection Based on Age-Stage, Two-Sex Life Table. National Chung Hsing University; Taichung, Taiwan: 2020. [Google Scholar]
- 18.Goodman D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982;119:803–823. doi: 10.1086/283956. [DOI] [Google Scholar]
- 19.Huang Y.-B., Chi H. Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: A case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) Agric. For. Entomol. 2012;61:37–45. [Google Scholar]
- 20.Göldel B., Lemic D., Bažok R. Alternatives to synthetic insecticides in the control of the colorado potato beetle (Leptinotarsa decemlineata Say) and their environmental benefits. Agriculture. 2020;10:611. doi: 10.3390/agriculture10120611. [DOI] [Google Scholar]
- 21.Sánchez-Bayo F., Tennekes H.A., Goka K. Insecticides-Development of Safer and More Effective Technologies. InTech; Rijeka, Croatia: 2013. Impact of systemic insecticides on organisms and ecosystems; pp. 365–414. [Google Scholar]
- 22.Clarke B.S., Jewess P.J. The inhibition of chitin synthesis in Spodoptera littoralis larvae by flufenoxuron, teflubenzuron and diflubenzuron. Pest Sci. 1990;28:377–388. doi: 10.1002/ps.2780280405. [DOI] [Google Scholar]
- 23.Rodríguez Enríquez C.L., Pineda S., Figueroa J.I., Schneider M.I., Martínez A.M. Toxicity and sublethal effects of methoxyfenozide on Spodoptera exigua (Lepidoptera: Noctuidae) J. Econ. Entomol. 2010;103:662–667. doi: 10.1603/EC09244. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoudvand M., Abbasipour H., Garjan A.S., Bandani A.R. Sublethal effects of hexaflumuron on development and reproduction of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae) Insect Sci. 2011;18:689–696. doi: 10.1111/j.1744-7917.2011.01411.x. [DOI] [Google Scholar]
- 25.Biddinger D., Hull L. Sublethal effects of selected insecticides on growth and reproduction of a laboratory susceptible strain of tufted apple bud moth (Lepidoptera: Tortricidae) J. Econ. Entomol. 1999;92:314–324. doi: 10.1093/jee/92.2.314. [DOI] [Google Scholar]
- 26.Josan A., Singh G. Sublethal effects of lufenuron on the diamondback moth, Plutella xylostella (Linnaeus) Insect Sci. Appl. 2000;20:303–308. doi: 10.1017/S1742758400015666. [DOI] [Google Scholar]
- 27.Meng Q.-W., Wang J.-J., Shi J.-F., Guo W.-C., Li G.-Q. Effect of teflubenzuron ingestion on larval performance and chitin content in Leptinotarsa decemlineata. Am. J. Potato Res. 2018;95:463–472. doi: 10.1007/s12230-018-9646-0. [DOI] [Google Scholar]
- 28.Xu C., Zhang Z., Cui K., Zhao Y., Han J., Liu F., Mu F. Effects of sublethal concentrations of cyantraniliprole on the development, fecundity and nutritional physiology of the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae) PLoS ONE. 2016;11:e0156555. doi: 10.1371/journal.pone.0156555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanzubil P.B., McCaffery A.R. Effects of Azadirachtin on reproduction in the African armyworm (Spodoptera exempta) Entomol. Exp. Appl. 1990;57:115–121. doi: 10.1111/j.1570-7458.1990.tb01422.x. [DOI] [Google Scholar]
- 30.Adel M.M., Sehnal F. Azadirachtin potentiates the action of ecdysteroid agonist RH-2485 in Spodoptera littoralis. J. Insect Physiol. 2000;46:267–274. doi: 10.1016/S0022-1910(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 31.Pineda S., Martínez A.M., Figueroa J.I., Schneider M.I., Del Estal P., Viñuela E., Gómez B., Smagghe G., Budia F. Influence of azadirachtin and methoxyfenozide on life parameters of Spodoptera littoralis (Lepidoptera: Noctuidae) J. Econ. Entomol. 2009;102:1490–1496. doi: 10.1603/029.102.0413. [DOI] [PubMed] [Google Scholar]
- 32.Guo L., Desneux N., Sonoda S., Liang P., Han P., Gao X.-W. Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Prot. 2013;48:29–34. doi: 10.1016/j.cropro.2013.02.009. [DOI] [Google Scholar]
- 33.Ahmad S., Ansari M.S. Acute toxicity and sub-lethal effects of a pyrethroid (cypermethrin) on survival, development and fitness of Helicoverpa armigera. Arch. Phytopathol. Plant Prot. 2013;46:1726–1739. doi: 10.1080/03235408.2013.774809. [DOI] [Google Scholar]
- 34.Song L., Zhang J. Sublethal effects of indoxacarb and beta-cypermethrin on Plutella xylostella (Lepidoptera: Plutellidae) Acta Entomol. Sin. 2013;56:521–529. [Google Scholar]
- 35.Quan L.-F., Qiu G.-S., Zhang H.-J., Sun L.-N., Li Y.-Y., Yan W.-T. Sublethal concentration of beta-cypermethrin influences fecundity and mating behavior of Carposina sasakii (Lepidoptera: Carposinidae) adults. J. Econ. Entomol. 2016;109:2196–2204. doi: 10.1093/jee/tow170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the M.Z.