Abstract
Chagas disease is a neglected tropical disease caused by the protozoan Trypanosoma cruzi and transmitted mainly by members of the subfamily Triatominae. There are currently 157 species, grouped into 18 genera and five tribes. Most descriptions of triatomine species are based on classical taxonomy. Facing evolutionary (cryptic speciation and phenotypic plasticity) and taxonomic (more than 190 synonymizations) problems, it is evident that integrative taxonomy studies are an important and necessary trend for this group of vectors. Almost two-and-a-half centuries after the description of the first species, we present for the first time the state-of-the-art taxonomy of the whole subfamily, covering from the initial classic studies to the use of integrative taxonomy.
Keywords: Triatominae, classical taxonomy, molecular taxonomy, integrative taxonomy
1. Triatominae: The Vectors of Chagas Disease
Chagas disease is a neglected tropical disease caused by the protozoan Trypanosoma cruzi (Chagas, 1909) (Kinetoplastida, Trypanosomatidae) [1]. This disease is found mainly in 21 Latin American countries, where it is mostly vector-borne, more specifically by members of the subfamily Triatominae (Hemiptera, Reduviidae) [1]. Triatomines or kissing bugs are hematophagous insects that have a habit of defecating during or after the blood meal—if they are infected with T. cruzi, they release the parasite in the feces/urine [1]. An estimated 8 million people are infected worldwide, and more than 65 million people at risk of acquiring the disease, which causes more than 12,000 deaths per year, the vector control being the most useful method to prevent new infections [1,2].
There are currently 157 species (154 extant species and three fossils), grouped into 18 genera and five tribes (Table 1) [3,4,5,6,7], being all potential vectors of T. cruzi. Taxonomic studies of Triatominae started in the 18th century with the description of Triatoma rubrofasciata (De Geer, 1773) (as Cimex rubro-fasciatus) [8]. Almost two and a half centuries after the description of the first species, we presented for—the first time—a review of the state-of-the-art of taxonomy of the whole subfamily, covering from the initial classic studies to the use of integrative taxonomy, a term formally introduced only in 2005 to describe taxa by integrating information from different data and methodologies [9,10].
Table 1.
Tribes, genera, and number of species that make up the subfamily Triatominae.
| Tribe | Genus | Species (n) |
|---|---|---|
| Alberproseniini | Alberprosenia | 2 |
| Bolboderini | Belminus | 9 |
| Bolbodera | 1 | |
| Microtriatoma | 2 | |
| Parabelminus | 2 | |
| Cavernicolini | Cavernicola | 2 |
| Rhodniini | Psammolestes | 3 |
| Rhodnius | 21 | |
| Triatomini | Dipetalogaster | 1 |
| Eratyrus | 2 | |
| Hermanlentia | 1 | |
| Linshcosteus | 6 | |
| Mepraia | 3 | |
| Nesotriatoma | 3 | |
| Panstrongylus | 15 | |
| Paratriatoma | 2 | |
| Triatoma | 81 | |
| Paleotriatoma | 1 | |
| Total | 157 |
2. Applications and Limitations of Triatominae Taxonomic Studies
For 225 years (1773–1998), the descriptions of triatomine species have been based only on studies of classical taxonomy (using descriptive morphology, comparative morphology, and/or morphometry) (Table 2). Although these analyses are imperative and are present in the description of all species of the subfamily Triatominae (Table 2), in the last decade, other approaches (such as biochemical [5,11], cytogenetic [5,12], phylogenetic [5,13,14,15,16,17] and/or of reproductive barriers [5]) started to be combined with the characterization of morphology and/or morphometry, employing the integrative taxonomy in the study of these insect vectors (Table 2).
Table 2.
Species, taxonomic tools, and taxonomic classification used in the description of Triatominae taxa.
| Species | Morphology and Morphometry | Chemotaxonomy | Cytotaxonomy | Experimental Crosses | Phylogenetic Systematics and Molecular Taxonomy | Taxonomy | References | |
|---|---|---|---|---|---|---|---|---|
| 1 | Triatoma rubrofasciata (De Geer, 1773) | X | Classical taxonomy | De Geer [8] | ||||
| 2 | Triatoma dimidiata (Latreille, 1811) | X | Classical taxonomy | Latreille [21] | ||||
| 3 | Panstrongylus geniculatus (Latreille, 1811) | X | Classical taxonomy | Latreille [21] | ||||
| 4 | Triatoma infestans (Klug, 1834) | X | Classical taxonomy | Klug [22] | ||||
| 5 | Triatoma phyllosomus (Burmeister, 1835) | X | Classical taxonomy | Burmeister [23] | ||||
| 6 | Panstrongylus megistus (Burmeister, 1835) | X | Classical taxonomy | Burmeister [23] | ||||
| 7 | Triatoma rubrovaria (Blanchard, 1846) | X | Classical taxonomy | Blanchard [24] | ||||
| 8 | Triatoma maculata (Erichson, 1848) | X | Classical taxonomy | Erichson [25] | ||||
| 9 | Triatoma mexicana (Herrich-Schaeffer, 1848) | X | Classical taxonomy | Herrich-Schaeffer [26] | ||||
| 10 | Triatoma sanguisuga (Leconte, 1855) | X | Classical taxonomy | Leconte [27] | ||||
| 11 | Belminus rugulosus (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 12 | Eratyruscuspidatus (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 13 | Eratyrusmucronatus (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 14 | Rhodnius nasutus (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 15 | Rhodnius prolixus (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 16 | Triatoma circummaculata (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 17 | Triatoma gerstaeckeri (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 18 | Paratriatoma lecticularia (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 19 | Triatoma sordida (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 20 | Triatoma vitticeps (Stål, 1859) | X | Classical taxonomy | Stål [28] | ||||
| 21 | Triatoma recurva (Stål, 1868) | X | Classical taxonomy | Stål [29] | ||||
| 22 | Triatoma venosa (Stål, 1872) | X | Classical taxonomy | Stål [30] | ||||
| 23 | Triatoma pallidipennis (Stål, 1872) | X | Classical taxonomy | Stål [30] | ||||
| 24 | Rhodnius pictipes (Stål, 1872) | X | Classical taxonomy | Stål [30] | ||||
| 25 | Triatoma nigromaculata (Stål, 1872) | X | Classical taxonomy | Stål [30] | ||||
| 26 | Panstrongylus lignarius (Walker, 1873) | X | Classical taxonomy | Walker [31] | ||||
| 27 | Panstrongylus guentheri (Berg, 1879) | X | Classical taxonomy | Berg [32] | ||||
| 28 | Triatoma rubida (Uhler, 1894) | X | Classical taxonomy | Uhler [33] | ||||
| 29 | Dipetalogaster maxima (Uhler, 1894) | X | Classical taxonomy | Uhler [33] | ||||
| 30 | Triatoma protracta (Uhler, 1894) | X | Classical taxonomy | Uhler [33] | ||||
| 31 | Panstrongylus rufotuberculatus (Champion, 1899) | X | Classical taxonomy | Champion [34] | ||||
| 32 | Triatoma migrans (Breddin, 1903) | X | Classical taxonomy | Breddin [35] | ||||
| 33 | Linshcosteuscarnifex (Distant, 1904) | X | Classical taxonomy | Distant [36] | ||||
| 34 | Bolbodera scabrosa (Valdés, 1910) | X | Classical taxonomy | Valdés [37] | ||||
| 35 | Nesotriatoma flavida (Neiva, 1911) | X | Classical taxonomy | Neiva [38] | ||||
| 36 | Psammolestes coreodes (Bergroth, 1911) | X | Classical taxonomy | Bergroth [39] | ||||
| 37 | Panstrongylus howardi (Neiva, 1911) | X | Classical taxonomy | Neiva [40] | ||||
| 38 | Triatoma brasiliensis (Neiva, 1911) | X | Classical taxonomy | Neiva [41] | ||||
| 39 | Triatoma neotomae (Neiva, 1911) | X | Classical taxonomy | Neiva [42] | ||||
| 40 | Triatoma indictiva (Neiva, 1912) | X | Classical taxonomy | Neiva [43] | ||||
| 41 | Triatoma platensis (Neiva, 1913) | X | Classical taxonomy | Neiva [44] | ||||
| 42 | Rhodnius brethesi (Matta, 1919) | X | Classical taxonomy | Matta [45] | ||||
| 43 | Panstrongylus lutzi (Neiva & Pinto, 1923) | X | Classical taxonomy | Neiva and Pinto [46] | ||||
| 44 | Rhodnius domesticus (Neiva & Pinto, 1923) | X | Classical taxonomy | Neiva and Pinto [47] | ||||
| 45 | Triatoma melanocephala (Neiva & Pinto, 1923) | X | Classical taxonomy | Neiva and Pinto [48] | ||||
| 46 | Triatoma bouvieri (Larrousse, 1924) | X | Classical taxonomy | Larrousse [49] | ||||
| 47 | Triatoma petrocchiae (Pinto & Barreto, 1925) | X | Classical taxonomy | Pinto and Barreto [50] | ||||
| 48 | Psammolestes arthuri (Pinto, 1926) | X | Classical taxonomy | Pinto [51] | ||||
| 49 | Triatoma carrioni (Larrousse, 1926) | X | Classical taxonomy | Larrousse [52] | ||||
| 50 | Triatoma tibiamaculata (Pinto, 1926) | X | Classical taxonomy | Pinto [53] | ||||
| 51 | Rhodnius robustus (Larrousse, 1927) | X | Classical taxonomy | Larrousse [54] | ||||
| 52 | Panstrongylus chinai (Del Ponte, 1929) | X | Classical taxonomy | Del Ponte [55] | ||||
| 53 | Triatoma breyeri (Del Ponte, 1929) | X | Classical taxonomy | Del Ponte [55] | ||||
| 54 | Triatoma eratyrusiformis (Del Ponte, 1929) | X | Classical taxonomy | Del Ponte [55] | ||||
| 55 | Triatoma limai (Del Ponte, 1929) | X | Classical taxonomy | Del Ponte [55] | ||||
| 56 | Triatoma patagonica (Del Ponte, 1929) | X | Classical taxonomy | Del Ponte [55] | ||||
| 57 | Rhodnius pallescens (Barber, 1932) | X | Classical taxonomy | Barber [56] | ||||
| 58 | Triatoma leopoldi (Schoudeten, 1933) | X | Classical taxonomy | Schoudeten [57] | ||||
| 59 | Mepraia spinolai (Porter, 1934) | X | Classical taxonomy | Porter [58] | ||||
| 60 | Cavernicola pilosa (Barber, 1937) | X | Classical taxonomy | Barber [59] | ||||
| 61 | Paratriatoma hirsuta (Barber, 1938) | X | Classical taxonomy | Barber [60] | ||||
| 62 | Triatoma longipennis (Usinger, 1939) | X | Classical taxonomy | Usinger [61] | ||||
| 63 | Triatoma picturatus (Usinger, 1939) | X | Classical taxonomy | Usinger [61] | ||||
| 64 | Panstrongylus humeralis (Usinger, 1939) | X | Classical taxonomy | Usinger [61] | ||||
| 65 | Triatoma barberi (Usinger, 1939) | X | Classical taxonomy | Usinger [61] | ||||
| 66 | Triatoma incrassata (Usinger, 1939) | X | Classical taxonomy | Usinger [61] | ||||
| 67 | Triatoma nitida (Usinger, 1939) | X | Classical taxonomy | Usinger [61] | ||||
| 68 | Triatoma oliveirai (Neiva et al., 1939) | X | Classical taxonomy | Neiva et al. [62] | ||||
| 69 | Triatoma arthurneivai (Lent & Martins, 1940) | X | Classical taxonomy | Lent and Martins [63] | ||||
| 70 | Triatoma hegneri (Mazzotti, 1940) | X | Classical taxonomy | Mazzotti [64] | ||||
| 71 | Triatoma peninsularis (Usinger, 1940) | X | Classical taxonomy | Usinger [65] | ||||
| 72 | Triatoma mazzottii (Usinger, 1941) | X | Classical taxonomy | Usinger [66] | ||||
| 73 | Triatoma melanica (Neiva & Lent, 1941) | X | Classical taxonomy | Neiva and Lent [67] | ||||
| 74 | Panstrongylus tupynambai (Lent, 1942) | X | Classical taxonomy | Lent [68] | ||||
| 75 | Parabelminus carioca (Lent, 1943) | X | Classical taxonomy | Lent [69] | ||||
| 76 | Panstrongylus diasi (Pinto & Lent, 1946) | X | Classical taxonomy | Pinto and Lent [70] | ||||
| 77 | Triatoma delpontei (Romaña & Abalos, 1947) | X | Classical taxonomy | Romaña and Abalos [71] | ||||
| 78 | Triatoma guasayana (Wygodzinsky & Abalos, 1949) | X | Classical taxonomy | Wygodzinsky and Abalos [72] | ||||
| 79 | Triatoma dispar (Lent, 1950) | X | Classical taxonomy | Lent [73] | ||||
| 80 | Triatoma wygodzinskyi (Lent, 1951a) | X | Classical taxonomy | Lent [74] | ||||
| 81 | Microtriatoma trinidadensis (Lent, 1951b) | X | Classical taxonomy | Lent [75] | ||||
| 82 | Triatoma amicitiae (Lent, 1951c) | X | Classical taxonomy | Lent [76] | ||||
| 83 | Rhodnius neivai (Lent, 1953) | X | Classical taxonomy | Lent [77] | ||||
| 84 | Triatoma matogrossensis (Leite & Barbosa, 1953) | X | Classical taxonomy | Leite and Barbosa [78] | ||||
| 85 | Triatoma pugasi (Lent, 1953b) | X | Classical taxonomy | Lent [79] | ||||
| 86 | Rhodnius neglectus (Lent, 1954) | X | Classical taxonomy | Lent [80] | ||||
| 87 | Belminus costaricencis (Herrer et al., 1954) | X | Classical taxonomy | Herrer et al. [81] | ||||
| 88 | Belminus peruvianus (Herrer et al., 1954) | X | Classical taxonomy | Herrer et al. [81] | ||||
| 89 | Rhodnius ecuadoriensis (Lent & León, 1958) | X | Classical taxonomy | Lent and León [82] | ||||
| 90 | Triatoma costalimai (Verano & Galvão, 1958) | X | Classical taxonomy | Verano and Galvão [83] | ||||
| 91 | Nesotriatoma obscura (Maldonado & Farr, 1962) | X | Classical taxonomy | Maldonado and Farr [84] | ||||
| 92 | Triatoma sinaloensis (Ryckman, 1962) | X | Classical taxonomy | Ryckman [85] | ||||
| 93 | Triatoma pseudomaculata (Corrêa & Espínola, 1964) | X | Classical taxonomy | Corrêa and Espínola [86] | ||||
| 94 | Psammolestes tertius (Lent & Jurberg, 1965) | X | Classical taxonomy | Lent and Jurberg [87] | ||||
| 95 | Triatoma sinica (Hsiao, 1965) | X | Classical taxonomy | Hsiao [88] | ||||
| 96 | Triatoma williami (Galvão et al., 1965) | X | Classical taxonomy | Galvão et al. [89] | ||||
| 97 | Triatoma bahiensis (Sherlock & Serafim, 1967) | X | Classical taxonomy | Sherlock and Serafim [90] | ||||
| 98 | Triatoma deaneorum (Galvão et al., 1967) | X | Classical taxonomy | Galvão et al. [91] | ||||
| 99 | Triatoma garciabesi (Carcavallo et al., 1967) | X | Classical taxonomy | Carcavallo et al. [92] | ||||
| 100 | Triatoma lenti (Sherlock & Serafim, 1967) | X | Classical taxonomy | Sherlock and Serafim [90] | ||||
| 101 | Panstrongylus lenti (Galvão & Palma, 1968) | X | Classical taxonomy | Galvão and Palma [93] | ||||
| 102 | Triatoma ryckmani (Zeledón & Ponce, 1972) | X | Classical taxonomy | Zeledón and Ponce [94] | ||||
| 103 | Rhodnius amazonicus (Almeida et al., 1973) | X | Classical taxonomy | Almeida et al. [95] | ||||
| 104 | Linshcosteusconfumus (Ghauri, 1976) | X | Classical taxonomy | Ghauri [96] | ||||
| 105 | Linshcosteuscostalis (Ghauri, 1976) | X | Classical taxonomy | Ghauri [96] | ||||
| 106 | Rhodnius dalessandroi (Carcavallo & Barreto, 1976) | X | Classical taxonomy | Carcavallo and Barreto [97] | ||||
| 107 | Alberproseniagoyovargasi (Martínez & Carcavallo, 1977) | X | Classical taxonomy | Martínez and Carcavallo [98] | ||||
| 108 | Rhodnius paraensis (Sherlock et al., 1977) | X | Classical taxonomy | Sherlock et al. [99] | ||||
| 109 | Triatoma cavernicola (Else & Cheong, 1977) | X | Classical taxonomy | Else et al. [100] | ||||
| 110 | Belminus herreri (Lent & Wygodzinsky, 1979) | X | Classical taxonomy | Lent and Wygodzinsky [101] | ||||
| 111 | Linshcosteuschota (Lent & Wygodzinsky, 1979) | X | Classical taxonomy | Lent and Wygodzinsky [101] | ||||
| 112 | Linshcosteuskali (Lent & Wygodzinsky, 1979) | X | Classical taxonomy | Lent and Wygodzinsky [101] | ||||
| 113 | Microtriatoma borbai (Lent & Wygodzinsky, 1979) | X | Classical taxonomy | Lent and Wygodzinsky [101] | ||||
| 114 | Parabelminus yurupucu (Lent & Wygodzinsky, 1979) | X | Classical taxonomy | Lent and Wygodzinsky [101] | ||||
| 115 | Triatoma guazu (Lent & Wygodzinsky, 1979) | X | Classical taxonomy | Lent and Wygodzinsky [101] | ||||
| 116 | Alberproseniamalheiroi (Serra et al., 1980) | X | Classical taxonomy | Serra et al. [102] | ||||
| 117 | Triatoma brailovskyi (Martínez et al., 1984) | X | Classical taxonomy | Martínez et al. [103] | ||||
| 118 | Cavernicola lenti (Barrett & Arias, 1985) | X | Classical taxonomy | Barrett and Arias [104] | ||||
| 119 | Triatoma bolivari (Carcavallo et al., 1987) | X | Classical taxonomy | Carcavallo et al. [105] | ||||
| 120 | Hermanlentia matsunoi (Fernández-Loayza, 1989) | X | Classical taxonomy | Fernández-Loayza [106] | ||||
| 121 | Rhodnius stali (Lent et al., 1993) | X | Classical taxonomy | Lent et al. [107] | ||||
| 122 | Belminus pittieri (Osuna & Ayala, 1993) | X | Classical taxonomy | Osuna and Ayala [108] | ||||
| 123 | Triatoma gomeznunezi (Martínez et al., 1994) | X | Classical taxonomy | Martínez et al. [109] | ||||
| 124 | Belminus laportei (Lent et al., 1995) | X | Classical taxonomy | Lent et al. [110] | ||||
| 125 | Mepraia gajardoi (Frias et al., 1998) | X | X | X | Integrative taxonomy | Frias et al. [111] | ||
| 126 | Triatoma carcavalloi (Jurberg et al., 1998) | X | Classical taxonomy | Jurberg et al. [112] | ||||
| 127 | Triatoma jurbergi (Carcavallo et al., 1998) | X | Classical taxonomy | Carcavallo et al. [113] | ||||
| 128 | Triatoma bassolsae (Alejandre Aguilar et al., 1999) | X | Classical taxonomy | Aguilar et al. [114] | ||||
| 129 | Rhodnius colombiensis (Mejia et al., 1999) | X | Classical taxonomy | Mejia et al. [115] | ||||
| 130 | Triatoma baratai (Carcavallo & Jurberg, 2000) | X | Classical taxonomy | Carcavallo and Jurberg [116] | ||||
| 131 | Rhodnius milesi (Carcavallo et al., 2001) | X | Classical taxonomy | Valente et al. [117] | ||||
| 132 | Triatoma klugi (Carcavallo et al., 2001) | X | Classical taxonomy | Carcavallo et al. [118] | ||||
| 133 | Linshcosteuskarupus (Galvão et al., 2002) | X | Classical taxonomy | Galvão et al. [119] | ||||
| 134 | Triatoma sherlocki (Papa et al., 2002) | X | Classical taxonomy | Papa et al. [120] | ||||
| 135 | Triatoma vandae (Carcavallo et al., 2002) | X | Classical taxonomy | Carcavallo [121] | ||||
| 136 | Triatoma dominicana (Ponair Jr., 2005) | X | Classical taxonomy | Ponair Jr. [122] | ||||
| 137 | Belminus corredori (Galvão & Angulo, 2006) | X | Classical taxonomy | Galvão and Ângulo [123] | ||||
| 138 | Belminus ferroae (Sandoval et al., 2007) | X | Classical taxonomy | Sandoval et al. [124] | ||||
| 139 | Panstrongylus mitarakaensis (Bérenger & Blanchet, 2007) | X | Classical taxonomy | Bérenger and Blanchet [125] | ||||
| 140 | Triatoma boliviana (Martinez et al., 2007) | X | Classical taxonomy | Martinez et al. [126] | ||||
| 141 | Triatoma juazeirensis (Costa & Felix, 2007) | X | Classical taxonomy | Costa and Felix [127] | ||||
| 142 | Panstrongylus martinezorum (Ayala, 2009) | X | Classical taxonomy | Ayala [128] | ||||
| 143 | Rhodnius zeledoni (Jurberg et al., 2009) | X | Classical taxonomy | Jurberg et al. [129] | ||||
| 144 | Mepraia parapatrica (Frías-Lasserre, 2010) | X | X | Integrative taxonomy | Frías-Lasserre [12] | |||
| 145 | Rhodnius montenegrensis (Rosa et al., 2012) | X | X | Integrative taxonomy | Rosa et al. [13] | |||
| 146 | Panstrongylus hispaniolae (Ponair Jr., 2013) | X | Classical taxonomy | Ponair Jr. [130] | ||||
| 147 | Rhodnius barretti (Abad-Franch et al., 2013) | X | X | Integrative taxonomy | Abad-Franch et al. [14] | |||
| 148 | Triatoma jatai (Gonçalves et al., 2013) | X | Classical taxonomy | Gonçalves et al. [131] | ||||
| 149 | Triatoma pintodiasi (Jurberg et al., 2013) | X | X | Integrative taxonomy | Jurberg et al. [11] | |||
| 150 | Rhodnius marabaensis (Souza et al., 2017) | X | X | Integrative taxonomy | Souza et al. [15] | |||
| 151 | Nesotriatoma confusa (Oliveira et al., 2018) | X | Classical taxonomy | Oliveira et al. [132] | ||||
| 152 | Triatoma mopan (Dorn et al., 2018) | X | X | Integrative taxonomy | Dorn et al. [16] | |||
| 153 | Paleotriatoma metaxytaxa (Poinar Jr., 2019 ) | X | Classical taxonomy | Poinar Jr. [133] | ||||
| 154 | Triatoma huehuetenanguensis (Lima-Cordon et al., 2019) | X | X | Integrative taxonomy | Lima-Cordon et al. [17] | |||
| 155 | Triatoma rosai (Alevi et al., 2020) | X | X | X | X | X | Integrative taxonomy | Alevi et al. [5] |
| 156 | Rhodnius micki (Zhao et al., 2021) | X | Classical taxonomy | Zhao et al. [6] | ||||
| 157 | Belminus santosmalletae (Dale et al., 2021) | X | Classical taxonomy | Dale et al. [7] |
More than 190 synonymization acts occurred in the subfamily Triatominae [18,19], with the majority of synonymized taxa being described from classical taxonomy. The use of combined analyses for the characterization of a taxon greatly reduces the chances of synonymization (although it does not make it impossible [19,20]). Based on the synonymization events and the importance of multi-analyses for the characterization of a taxon, we will discuss the current issues, applications, and limitations of classical, molecular, and integrative taxonomy.
2.1. Classical Taxonomy
Classical taxonomy underlies most taxonomic studies of species description in the subfamily Triatominae (Table 2). The morphological and morphometric studies applied in the last described taxa are: morphological study of the head, thorax, abdomen, and male and female genitalia (with optical microscopy (OM) and/or scanning electronic microscopy (SEM)), and morphometric study of the head, thorax, abdomen and appendices (using OM) [5,6,7,15,16,17,132].
Although the use of morphological and morphometric characters is essential to describe a new taxon (since the diagnosis of the species needs to be made based on specimens that will be deposited, such as vouchers, in entomological collections), evolutionary events of cryptic speciation [14] and phenotypic plasticity [14] present in the subfamily Triatominae can make it difficult to diagnose a taxon only by morphological studies. Classic examples of this can be seen in the genus Rhodnius Stål, 1859: R. montenegrensis Rosa et al., 2012 [13] and R. marabaensis Souza et al., 2017 [15] represent two of the four paraphyletic strains of R. robustus Larrousse, 1927 [134,135] (the application of integrative taxonomy allowed description of the species from specimens initially characterized as R. robustus [136]). On the other hand, was demonstrated that R. taquarussiensis Rosa et al., 2017 (species described by integrative taxonomy [20]) represented only an intraspecific polymorphism of R. neglectus Lent, 1954 [19] (from studies of molecular taxonomy combined with experimental crosses it was possible to synonymize the species [19]).
Morphological convergence events can also hinder the classic taxonomy of these vectors [129]. The paraphyletic genus Triatoma Laporte, 1832 needs several studies from a taxonomic and systematic point of view [137]. Triatoma tibiamaculata (Pinto, 1926), for example, is a species that has morphological characteristics that bring it together and groups it (until now) as a Triatoma [138]. However, the generic status of this vector has been questioned several times [134,137,138]—since it presents cytogenetic [139], structural [140] and phylogenetic [137,138] characteristics that bring it closer to Panstrongylus (which highlights the importance of studies with integrative taxonomy).
2.2. Molecular Taxonomy
The first phylogenetic trees with molecular markers were published only in 1998 [141], giving rise to the phylogenetic systematics and molecular taxonomy of these vectors. Although no species of triatomine has been described by molecular taxonomy (Table 2), the combination of phylogenetic analyses with morphological and morphometric studies in species description studies (integrative taxonomy) has been a trend in the last decade [5,13,14,15,16,17] (Table 2), since it provides greater reliability of the specific status of the taxa and allows, above all, to understand the evolutionary history of the species.
In addition to the contributions mentioned above, molecular taxonomy and phylogenetic systematics allowed the evaluation and re-validation of the taxonomic status of some species: reinclusion of Linshcosteus Distant, 1904 genus in Triatomini tribe (extinguishing the Linshcosteini tribe) [30]; inclusion of Psammolestes Bergroth, 1911 species in the genus Rhodnius [30] (proposal not accepted by the scientific community due to the differences that support the generic status of Psammolestes [17]); inclusion of the species T. flavida Neiva, 1911, and N. obscura Maldonado & Farr, 1962 in the genus Nesotriatoma Usinger, 1944 [142]; confirmation of the generic status of Nesotriatoma [132]; inclusion of species T. spinolai Porter, 1934, M. gajardoi Frias, Henry & Gonzalez, 1998, T. eratyrusiformis Del Ponte, 1929, and T. breyeri Del Ponte, 1929 in the genus Mepraia Mazza, Gajardo & Jörg, 1940 [142] (partially accepted suggestion, being the Mepraia genus currently composed of M. spinolai, M. gajardoi, and M. parapatrica Frías-Lasserre, 2010 [4,143]); confirmation of the generic status of Mepraia [137]; and inclusion of T. dimidiata (Latreille, 1811) in the Meccus Stål, 1859 genus (genus that later was considered invalid and the Meccus species started to be considered as Triatoma [137,144,145]).
Although the International Code of Zoological Nomenclature does not consider groupings of triatomines to be complexes or subcomplexes [146], Justi et al. [137] suggests that these groupings should represent monophyletic groups. In the genus Triatoma, for example, studies based on phylogenetic systematics evaluated the position of several species that had been grouped mainly by geographic distribution and morphological similarities and proposed regrouping and/or the creation of new monophyletic groups [137,147,148]. Species well defined as natural groups (monophyletic) are currently the T. brasiliensis [149,150], T. sordida [151], T. rubrovaria [151], T. infestans [137], and T. vitticeps [148] subcomplexes.
2.3. Integrative Taxonomy
The data integration in the integrative taxonomy can be done by cumulation or congruence [152]. The use of combined tools to delimit a species of triatomine occurred for the first time in 1998 by Frias et al. [111] who combined morphological, morphometric, cytogenetic, and reproductive barriers data to describe M. gajardoi (Table 2). However, only in the last decade has the integrative taxonomy has been more applied in the study of these vectors (Table 2).
This tendency to integrate different analyses to characterize a taxon, made it possible to resolve ancient taxonomic issues, such as the description by T. mopan Dorn et al. (2018) and T. huehuetenanguensis Lima-Cordón et al. (2019) from specimens initially characterized as T. dimidiata [16,17,153,154] and the recent description of T. rosai Alevi et al., 2020 from the allopatric population of T. sordida (Stål, 1859) from Argentina [5,155,156]. In addition, the specific status of T. bahiensis Sherlock & Serafim, 1967 (a species that for more than three decades has been synonymous with T. lenti Sherlock & Serafim, 1967 [101]) has been revalidated based on integrative taxonomy [149].
On the other hand, even if the integrative taxonomy provides more robustness in the characterization of the new taxa (decreasing the chance of synonymization), does not prevent this event can occur (as mentioned above for R. taquarussuensis which has been synonymous with R. neglectus Lent, 1954 [19]). Although morphological, morphometric, and cytogenetic intraspecific variation had been described in the genus Rhodnius [157,158], the description of R. taquarussuensis was based on these factors [20]. Thus, synonymization event occurred through phylogenetic analyses and experimental crosses [19]. We suggest that integrative taxonomy work should include molecular studies and, whenever possible, reproductive barriers to confirm the taxon specific status following the biological concept of species [159,160,161].
In general, most articles of description based on integrative taxonomy combine only morphological and morphometric data with molecular analyses (Table 2). However, it is worth mentioning that in 2020 the description of T. rosai was published based on morphometric, morphological, molecular data, and experimental crosses that have been combined with information from the literature about the species (cytogenetic data [155,156], electrophoresis pattern [155], cuticular hydrocarbons pattern [162], geometric morphometry [163], cycle, and average time of life [164,165,166] as well as geographic distribution [18,42,43,44,50,51]), becoming the most complete article of species description of the subfamily Triatominae [5].
3. Overview of Tools Applied to Taxonomic Studies of Triatomines
In addition to species descriptions, several taxonomic studies have been carried out to assess the specific status of valid species and, above all, to assist in the correct classification of Chagas disease vectors. Based on this, we will specifically discuss the application of each taxonomic tool.
3.1. Morphology and Comparative Morphology
As already mentioned above, morphological studies are applied to all formal species descriptions (Table 2). These analyses can characterize several structures that, in general, are compared and confirm the specific status of triatomines [5,6,11,12,13,14,15,16,17]. Studies with OM and SEM allow characterizing structures of the head, thorax, and abdomen. These analyses are very important for classical taxonomy and support the main dichotomous keys used for the correct identification of these vectors [101,167,168,169,170,171,172].
3.2. Morphometry
Like morphological studies, morphometric studies are also present in the description of all triatomines (at first, showing the size of specimens and structures and, later, by means of geometric morphometry [4]). These measurable data are very important from a taxonomic point of view, as a visual identification system was recently developed from morphometric data that has the potential to automate the identification of triatomines [173,174].
3.3. Chemotaxonomy
In 1964, Actis et al. [175] used, for the first time, biochemical studies with hemolymph protein electrophoresis to compare species of triatomines, giving rise to chemotaxonomy. Isoenzymes were applied to different species of Rhodnius [176], the T. brasiliensis subcomplex [177] and Mexican Triatoma [178]. However, recently, biochemical studies are rare from a taxonomic perspective; they contribute to the integrative taxonomy as shown by Jurberg et al. [11] and Alevi et al. [5] with the species descriptions of T. pintodiasi Jurberg et al., 2013 and T. rosai respectively.
3.4. Cytotaxonomy and Karyosystematic
Cytotaxonomy was started with Ueshima [179] by proposing the application of cytogenetic studies of chromosomes to differentiate morphologically related species. Later, the use of chromosomal analyses—such as karyotypes [180,181,182,183]—the constitutive heterochromatin pattern [156,184,185], the heterochromatin base pair composition [186,187,188], and the location of the nucleolar organizing region [139,156,189], assisted in the correct identification and classification of triatomines. Recently, dichotomous keys have been proposed based on cytogenetic data [190,191,192,193].
3.5. MALDI-TOF MS
Laroche et al. [194] used, for the first time, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis to differentiate triatomine species. The researchers were able to differentiate species from French Guiana by MALDI-TOF. Subsequently, Souza et al. [195] used these analyses to differentiate 12 species of the genus Rhodnius. Furthermore, Souza et al. [196] also differentiated the species of Cavernicola Barber, 1937.
3.6. Omics
In 2017, omics tools (transcriptomics) were used for the first time in taxonomic studies of triatomines to confirm the specific status of R. montenegrensis [197]. In 2019, Brito et al. [198] also validated the specific status of R. montenegrensis and confirmed that this species refers to strain II of the paraphyletic group of R. robustus.
4. Concluding Remarks
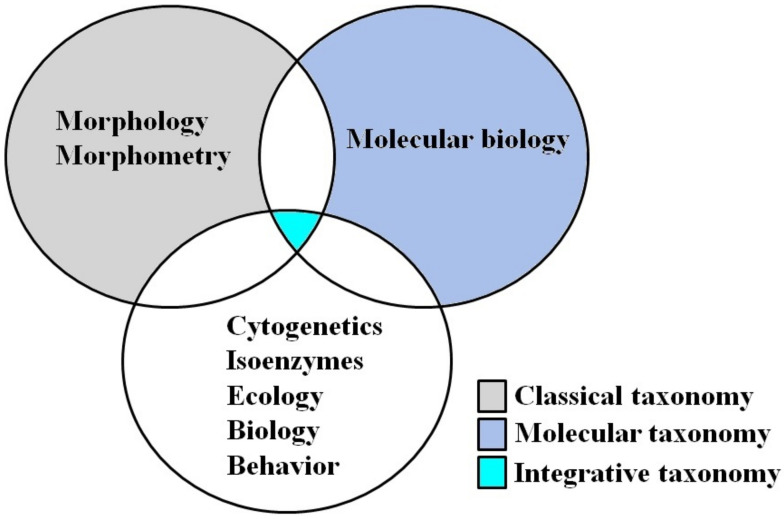
Classical taxonomy, over the last few decades, has been revitalized by integrative taxonomy leading to success in the identification and delimitation of new species through the use of multiple and complementary approaches. Most descriptions of triatomine species are based on classical taxonomy. Facing evolutionary (cryptic speciation and phenotypic plasticity) and taxonomic (more than 190 synonymizations) problems has indicated that it is evident that integrative taxonomy studies are an important and necessary trend for this group of vectors. However, from the synonymization of R. taquarussuensis (which was described through integrative taxonomy [20] and was later synonymized with R. neglectus [19]), it is evident that phylogenetic studies (molecular taxonomy) should be considered among the analyses used for the description of new species from the integrative taxonomy (Figure 1).
Figure 1.
Schematic representation of the integrative taxonomy of triatomines.
Author Contributions
Conceptualization, K.C.C.A., J.d.O., D.d.S.R. and C.G.; Writing—original draft preparation, K.C.C.A., J.d.O., D.d.S.R. and C.G.; Writing—review and editing, K.C.C.A., J.d.O., D.d.S.R. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation, Brazil (FAPESP) (Process number 2017/05015-7 and 2019/02145-2), the Coordination for the Improvement of Higher Education Personnel, Brazil (CAPES)—Finance Code 001 and the National Council for Scientific and Technological Development, Brazil (CNPq).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. [(accessed on 28 October 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/chagasdisease-(american-trypanosomiasis)
- 2.Pan American Health Organization. [(accessed on 26 November 2021)]. Available online: https://www.paho.org/en/topics/chagas-disease.
- 3.Justi S.A., Galvão C. The Evolutionary Origin of Diversity in Chagas Disease Vectors. Trends Parasit. 2017;33:42–52. doi: 10.1016/j.pt.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galvão C. Taxonomia dos Vetores da Doença de Chagas da Forma à Molécula, quase três séculos de história. In: Oliveira J., Alevi K.C.C., Camargo L.M.A., Meneguetti D.U.O., editors. Atualidades em Medicina Tropical no Brasil: Vetores. 1st ed. Stricto Sensu Editora; Rio Branco, Brasil: 2020. pp. 9–37. (In Portuguese) [Google Scholar]
- 5.Alevi K.C.C., Oliveira J., Garcia A.C.C., Cristal D.C., Delgado L.M.G., Bittinelli I.F., Reis Y.V., Ravazi A., Oliveira A.B.B., Galvão C., et al. Triatoma rosai sp. nov. (Hemiptera, Triatominae): A New Species of Argentinian Chagas Disease Vector Described Based on Integrative Taxonomy. Insects. 2020;11:830. doi: 10.3390/insects11120830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y., Galvão C., Cai W. Rhodnius micki, a new species of Triatominae (Hemiptera, Reduviidae) from Bolivia. ZooKeys. 2021;1012:71–93. doi: 10.3897/zookeys.1012.54779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale C., Justi S.A., Galvão C. Belminus santosmalletae (Hemiptera: Heteroptera: Reduviidae): New Species from Panama, with an Updated Key for Belminus Stål, 1859 Species. Insects. 2021;12:686. doi: 10.3390/insects12080686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Geer C. Mémoires pour Servir à l’Historie des Insectes. L.L. Grefing; Stockholm, Sweden: 1773. pp. 1–696. (In French) [Google Scholar]
- 9.Dayrat B. Towards integrative taxonomy. Biol. J. Linn. Soc. 2005;85:407–415. doi: 10.1111/j.1095-8312.2005.00503.x. [DOI] [Google Scholar]
- 10.Will K.W., Mishler B.D., Wheeler Q.D. The perils of DNA barcoding and the need of integrative taxonomy. System. Biol. 2005;54:844–851. doi: 10.1080/10635150500354878. [DOI] [PubMed] [Google Scholar]
- 11.Jurberg J., Cunha V., Cailleaux S., Raigorodschi R., Lima M.S., Rocha D.S., Moreira F.F.F. Triatoma pintodiasi sp. nov. do subcomplexo T. rubrovaria (Hemiptera, Reduviidae, Triatominae) Rev. Pan Amaz. Saúde. 2013;4:43–56. doi: 10.5123/S2176-62232013000100006. [DOI] [Google Scholar]
- 12.Frías-Lasserre D. A new species and karyotype variation in the bordering distribution of Mepraia spinolai (Porter) and Mepraia gajardoi Frías et al. (Hemiptera: Reduviidae: Triatominae) in Chile and its parapatric model of speciation. Neotrop. Entom. 2010;39:572–583. doi: 10.1590/S1519-566X2010000400017. [DOI] [PubMed] [Google Scholar]
- 13.Rosa J.A., Rocha C.S., Gardim S., Pinto M.C., Mendonça V.J., Ferreira Filho J.C.R., Carvalho E.C., Camargo L.M.A., Oliveira J., Nascimento J.D., et al. Description of Rhodnius montenegrensis n. sp. (Hemiptera: Reduviidae:Triatominae) from the state of Rondônia, Brazil. Zootaxa. 2012;347:62–76. doi: 10.11646/zootaxa.3478.1.8. [DOI] [Google Scholar]
- 14.Abad-Franch F., Pavan M.G., Jaramillo-O N., Palomeque F.S., Dale C., Chaverra D., Monteiro F. A Rhodnius barretti, a new species of Triatominae (Hemiptera: Reduviidae) from western Amazonia. Mem. Inst. Oswaldo Cruz. 2013;108:92–99. doi: 10.1590/0074-0276130434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Souza E.S., Von Atzingen N.C.N., Furtado M.B., Oliveira J., Nascimento J.D., Vendrami D.P., Gardim S., Rosa J.A. Description of Rhodnius marabaensis sp. n. (Hemiptera, Reduviidae, Triatominae) from Pará State, Brazil. ZooKeys. 2016;621:45–62. doi: 10.3897/zookeys.621.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorn P.L., Justi A.S., Dale C., Stevens L., Galvão C., Cordon R.L., Monroy C. Description of Triatoma mopan sp. n. from a cave in Belize (Hemiptera, Reduviidae, Triatominae) ZooKeys. 2018;775:69–95. doi: 10.3897/zookeys.775.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima-Cordón R.A., Monroy M.C., Stevens L., Rodas A., Rodas G.A., Dorni P.L., Justi A.S. Description of Triatoma huehuetenanguensis sp. n., a potential Chagas disease vector (Hemiptera, Reduviidae, Triatominae) ZooKeys. 2019;820:51–70. doi: 10.3897/zookeys.820.27258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvão C., Carcavallo R., Rocha D.S., Jurberg J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;202:1–36. doi: 10.11646/zootaxa.202.1.1. [DOI] [Google Scholar]
- 19.Nascimento J.D., Rosa J.A., Salgado-Roa F.C., Hernández C., Pardo-Diaz C., Alevi K.C.C., Ravazi A., Oliveira J., Azeredo-Oliveira M.T.V., Salazar C., et al. Taxonomical over splitting in the Rhodnius prolixus (Insecta: Hemiptera: Reduviidae) clade: Are R. taquarussuensis (da Rosa et al., 2017) and R. neglectus (Lent, 1954) the same species? PLoS ONE. 2019;14:e0213043. doi: 10.1371/journal.pone.0211285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosa J.A., Justino H.H.G., Nascimento J.D., Mendonça V.J., Rocha C.S., Carvalho D.B., Falcone R., Azeredo-Oliveira M.T.V., Alevi K.C.C., Oliveira J. A new species of Rhodnius from Brazil (Hemiptera, Reduviidae, Triatominae) ZooKeys. 2017;675:1–25. doi: 10.3897/zookeys.675.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latreille P.A. Insectes de líAmérique recueillis pendant le voyage de MM. In: Humboldt A., Bonpland A., editors. Voyage aux Régions Équinoxiales du Nouveau Continent. 1811. pp. 197–397. (In French) [Google Scholar]
- 22.Klug J.C.F. Reise um die Erde, in den Jahren 1830, 1831, und 1832, Ausgefürht von F.J.F.Meyen. Teil 1. C. W. Eichhoff; Berlin, Germany: 1834. (In German) [Google Scholar]
- 23.Burmeister H. Handbuch der Entomologie. T. Enslin; Berlin, Germany: 1835. p. 400. Tome 2, part 1. (In German) [Google Scholar]
- 24.Blanchard E. D’Orbigny, A. [1837–1846], Voyage dans L’Amérique Méridionale, Tome Sixiéme, 2.e Partie: Insectes. Chez. P. Bertrand; Paris, France: 1846. Hémiptères; pp. 218–222. (In French) [Google Scholar]
- 25.Erichson W.F. Schomburgk, R. Reisen in Britisch-GUIANA in der jahren 1840–1844 im auftrag Sr Majestat des Konings von Pruessen. Weber; Leipzig, Germany: 1848. Insecten; pp. 553–617. (In German) [Google Scholar]
- 26.Herrich-Schaeffer G.H.W. Die Wanzenartigen Insekten. C. H. Zehschen Buchhandlung; Nurnberg, Germany: 1848. p. 130. (In German) [Google Scholar]
- 27.Leconte J.L. Remarks on two species of American Cimex. Proc. Acad. Nat. Sci. Phila. 1855;7:404. [Google Scholar]
- 28.Stål C. Monographie der Gattung Conorhinus und Verwandten. Berliner Entomol Zeitschrift. 1859;3:99–117. doi: 10.1002/mmnd.18590030202. (In German) [DOI] [Google Scholar]
- 29.Stål C. Heteroptera. Kongliga Svenska Vetenskaps-Akademiens; Stockholm, Sweden: 1868. Hemiptera Fabriciana; Pars 1; pp. 1–148. (In Swedish) [Google Scholar]
- 30.Stål C. Kongliga Svenska Vetenskaps-Akademiens; Stockholm, Sweden: 1872. Enumeratio Hemipterorum; Pars 2; pp. 1–159. [Google Scholar]
- 31.Walker F. Catalogue of the Specimens of Hemiptera Heteroptera in the Collection of the British Museum. BM(NH); London, UK: 1873. p. 220. [Google Scholar]
- 32.Berg C. Hemiptera Argentina Enumeravit Speciesque Novas. P. E. Coni; Buenos Aires, Argentina: 1879. p. 316. [Google Scholar]
- 33.Uhler P.R. Observations upon the Heteropterous Hemiptera of Lower California, with description of new species. Proc. Calif. Acad. Sci. 1894;4:223–295. [Google Scholar]
- 34.Champion G.C. Insecta Rhynchota. Hemiptera-Heteroptera. In: Porter R.H., editor. Biologia Centrali-Americana. W. L. Distant; London, UK: 1899. p. 416. [Google Scholar]
- 35.Breddin G. Neue Paläotropische Reduviinen. Sber. Ges. Naturf. Freunde Berl. 1903;3:111–129. [Google Scholar]
- 36.Distant W.L. The Fauna of British India, Including Ceylon and Burma. Rhynchota. Vol. 2. Taylor & Francis; London, UK: 1904. 503p [Google Scholar]
- 37.Valdés P.R. Clasificación Gundlach de Hemipteros Cubanos, conforme a los ejemplares que existen en el Museo del Instituto de 2a enseñanza de La Habana. Anales de la Academia de Ciencias Médicas, Físicas y Naturales de la Habana. 1910;46:425–446. (In Spanish) [Google Scholar]
- 38.Neiva A. Notas de entomología médica. Duas novas espécies norte-americanas de hemípteros hematophagos. Brasil-Médico. 1911;25:421–422. (In Portuguese) [Google Scholar]
- 39.Bergroth E. A new genus of Reduviidae. Psyche. 1911;18:144–145. doi: 10.1155/1911/724151. [DOI] [Google Scholar]
- 40.Neiva A. Zwei neue Afrikanische Arten des Genus Triatoma (oder Conorhinus) Proc. Entomol. Soc. Wash. 1911;13:239–240. (In German) [Google Scholar]
- 41.Neiva A. Contribuição para o estudo dos hematophagos brasileiros e descrição de uma nova espécie de Triatoma. Brasil-Médico. 1911;25:461–462. (In Portuguese) [Google Scholar]
- 42.Neiva A. Notas de entomología médica. Três novas especies de reduvidas norte-americanas. Bras. Méd. 1911;25:441. (In Portuguese) [Google Scholar]
- 43.Neiva A. Notas de entomología médica e descripção de duas novas espécies de Triatomas norte-americanas. Bras. Méd. 1912;26:21–22. (In Portuguese) [Google Scholar]
- 44.Neiva A. Algunos datos sobre hemípteros hematófagos de la América del sur, con la descripción de una nueva especie. Anales del Museo Nacional de Historia Natural. 1913;24:195–198. (In Spanish) [Google Scholar]
- 45.Matta A. Um novo Reduvido do Amazonas, Rhodnius brethesi n. sp. Amaz. Méd. 1919;2:93–94. (In Portuguese) [Google Scholar]
- 46.Neiva A., Pinto C. Dos hemípteros hematophagos do Norte do Brasil com descrição de duas novas espécies. Bras. Méd. 1923;1923:73–76. (In Portuguese) [Google Scholar]
- 47.Neiva A., Pinto C. Estado actual dos conhecimentos sôbre o gênero Rhodnius Stål, com a descripção de uma nova espécie. Bras. Méd. 1923;37:20–24. (In Portuguese) [Google Scholar]
- 48.Neiva A., Pinto C. Representantes dos gêneros Triatoma Lap. e Rhodnius Stål, encontrados no Brasil Central e Sul; observações biológicos e descripção de uma nova espécie. Bras. Méd. 1923;37:84–86. (In Portuguese) [Google Scholar]
- 49.Larrousse F. Triatomes díAsie: Description díune nouvelle espèce Triatoma bouvieri n. sp. Annales de Parasitologie Humaine et Comparée. 1924;2:62–70. doi: 10.1051/parasite/1924021062. (In Spanish) [DOI] [Google Scholar]
- 50.Pinto C., Barreto J.B. Uma nova espécie de “barbeiro” do Brasil, (Triatoma petrochii n.s p.) Sci. Med. 1925;3:769. (In Portuguese) [Google Scholar]
- 51.Pinto C. Triatomideos da Venezuela, com a descripção de uma nova espécie do gênero Eutriatoma. Ann. Fac. Med. São Paulo. 1926;1:85–87. (In Portuguese) [Google Scholar]
- 52.Larrousse F. Description de deux espéces nouvelles du genre Triatoma: T. carrioni n. sp., et T. pintoi n. sp. Annales de Parasitologie Humaine et Comparée. 1926;4:136–139. doi: 10.1051/parasite/1926042136. (In Spanish) [DOI] [Google Scholar]
- 53.Pinto C. Eutriatoma tibiamaculata novo género e nova espécie, forma intermediaria entre Rhodnius e Triatoma. Sci. Med. 1926;3:133–136. (In Portuguese) [Google Scholar]
- 54.Larrousse F. Etude biologique et systématique du genre Rhodnius Stål (Hémiptères, Reduviidae) Annales de Parasitologie. 1927;5:63–88. (In French) [Google Scholar]
- 55.Del Ponte E. Algunas especies nuevas del género Triatoma Lap. Boletin de la Sociedad Entomológica Argentina. 1929;1:3–8. (In Spanish) [Google Scholar]
- 56.Barber H.G. A new species of Rhodnius from Panama (Hemiptera: Reduviidae) J. Wash. Acad. Sci. 1932;22:514–517. [Google Scholar]
- 57.Schoudeten H. Résultats scientifiques du voyage de LL. AA. RR. le Prince et la Princesse de Belgique. Hemiptera ñ Heteroptera. Memoires du Musee Royal dí Histoire Naturelle Belgique. 1933;4:43–70. (In French) [Google Scholar]
- 58.Porter C.E. Una Triatoma nueva chilena. Revista Chilena de Historia Natural. 1934;37:192–193. (In Spanish) [Google Scholar]
- 59.Barber H.G. A new bat-cave bug from Panama (Hemiptera-Heteroptera, Reduviidae) Proc. Entomol. Soc. Wash. 1937;39:60–63. [Google Scholar]
- 60.Barber H.G. A new genus and species of the subfamily Triatominae (Reduviidae: Hemiptera) Proc. Entomol. Soc. Wash. 1938;40:104–105. [Google Scholar]
- 61.Usinger R.L. Descriptions of new Triatominae with a key to genera (Hemiptera, Reduviidae) Univ. Calif. Publ. Entomol. 1939;7:33–56. [Google Scholar]
- 62.Neiva A., Pinto C., Lent H. Notas sobre triatomideos do Rio Grande do sul e descrição de uma nova espécie. Memórias do Instituto Oswaldo Cruz. 1939;34:607–610. doi: 10.1590/S0074-02761939000400009. (In Spanish) [DOI] [Google Scholar]
- 63.Lent H., Martins A.V. Estudos sôbre os triatomídeos do Estado de Minas Gerais, com descrição de uma espécie nova. Rev. Entomol. 1940;11:877–886. (In Portuguese) [Google Scholar]
- 64.Mazzottii L. Una nueva especie de Triatoma en Mexico. Cien Méx. 1940;1:22–23. (In Spanish) [Google Scholar]
- 65.Usinger R.L. A new Triatoma from Lower California (Hemiptera, Reduviidae) Pan Pacific Ent. 1940;16:73–74. [Google Scholar]
- 66.Usinger R.L. Notes and descriptions of neotropical Triatominae (Hemiptera, Reduviidae) Pan Pacific Ent. 1941;17:49–57. [Google Scholar]
- 67.Neiva A., Lent H. Sinopse dos Triatomideos. Rev. Ent. 1941;12:61–92. (In Spanish) [Google Scholar]
- 68.Lent H. Estudos sobre os triatomideos do Estado do Rio Grande do Sul, com descrição de uma espécie nova. Rev. Bras. Biol. 1942;2:219–231. (In Portuguese) [Google Scholar]
- 69.Lent H. Novo transmissor da doença de Chagas na cidade de Rio de Janeiro, D.F. Estudo dos gêneros Belminus Stål, 1859, Bolbodera Valdés, 1910 e descrição de Parabelminus carioca n.g., n. sp. (Hemiptera, Triatomidae) Memórias do Instituto Oswaldo Cruz. 1943;38:497–516. doi: 10.1590/S0074-02761943000300015. (In Portuguese) [DOI] [Google Scholar]
- 70.Pinto C., Lent H. Novo hemíptero hematófago do gênero Panstrongylus Berg, 1879. Rev. Bras. Biol. 1946;6:459–465. (In Portuguese) [Google Scholar]
- 71.Romaña C., Abalos J. Triatoma delpontei n. sp. (Hemiptera, Reduviidae) Anales del Inst. de Med. Reg. Tucumán. 1947;2:79–93. [Google Scholar]
- 72.Wygodzinsky P., Abalos J.W. Triatoma guasayana sp. n. (Triatominae, Reduviidae, Hemiptera) (Nota previa) Semana Médica. 1949;56:2. [Google Scholar]
- 73.Lent H. Nova espécie de Triatoma Laporte, 1833 (Hemiptera, Reduviidae) Rev. Bras. Biol. 1950;10:437–440. (In Portuguese) [Google Scholar]
- 74.Lent H. Novo Triatoma do Estado de Minas Gerais (Brasil) (Hemiptera, Reduviidae) Rev. Entom. 1951;22:349–352. (In Portuguese) [Google Scholar]
- 75.Lent H. Segunda espécie do gênero Bolbodera Valdés, 1910 (Hemiptera, Reduviidae) Rev. Bras. Biol. 1951;11:153–156. (In Portuguese) [Google Scholar]
- 76.Lent H. Triatominae das regiões Oriental, Australiana, Etiópica e Paleártica, com descrição de uma nova espécie (Hemiptera, Reduviidae) Rev. Bras. Biol. 1951;11:425–429. (In Portuguese) [Google Scholar]
- 77.Lent H. Um novo hemiptero hematófago da Venezuela (Reduviidae, Triatominae) Rev. Bras. Biol. 1953;13:169–172. (In Portuguese) [Google Scholar]
- 78.Leite I.C., Barbosa A. Triatoma (Eutriatoma) matogrossensis n sp. Bol. do Inst. Oswaldo Cruz. 1953;2:123–126. [PubMed] [Google Scholar]
- 79.Lent H. Nova espécie de Triatoma da Região Oriental (Hemiptera, Reduviidae) Rev. Bras. de Biol. 1953;13:315–319. (In Portuguese) [Google Scholar]
- 80.Lent H. Comentários sôbre o gênero Rhodnius Stål com descrição de uma nova espécie do Brasil (Hemiptera, Reduviidae) Rev. Bras. de Biol. 1954;14:237–247. (In Portuguese) [Google Scholar]
- 81.Herrer A. Contribución al conocimiento del género Belminus Stål, 1859 (Triatominae, Reduviidae, Hemiptera) Anales del Inst. de Med. Reg. Tucumán. 1954;4:85–105. (In Spanish) [Google Scholar]
- 82.Lent H., León L.A. Um novo Rhodnius Stål do Ecuador (Hemiptera, Reduviidae) Rev. Bras. Biol. 1958;18:181–185. (In Portuguese) [Google Scholar]
- 83.Verano O.T., Galvão A.B. Triatoma costalimai n. sp. Rev. Bras. Mal. Doenças Trop. 1958;10:199–205. [Google Scholar]
- 84.Maldonado J., Farr T.H. On some Jamaican Triatominae and Emesinae. Proc. Entomol. Soc. Wash. 1962;64:187–194. [Google Scholar]
- 85.Ryckman R.E. Biosystematics and hosts of the Triatoma protracta complex in NorthAmerica (Hemiptera: Reduviidae) (Rodentia: Cricetidae) Vol. 27. University of California Press; Oakland, CA, USA: 1962. pp. 93–240. [Google Scholar]
- 86.Correa R.R., Espínola H.N. Descrição de Triatoma pseudomaculata, nova espécie de triatomíneo de Sobral, Ceará (Hemiptera, Reduviidae) Arquivos de Higiene e Saude Publica. 1964;29:115–127. (In Portuguese) [PubMed] [Google Scholar]
- 87.Lent H., Jurberg J. O gênero Psammolestes Bergroth, 1911, com um estudo sôbre a genitália das espécies (Hemiptera, Reduviidae, Triatominae) Rev. Bras. Biol. 1965;25:349–376. (In Portuguese) [Google Scholar]
- 88.Hsaio T.Y. A new species of Triatoma Laporte (Hemiptera, Reduviidae) Acta Zootax. Sinica. 1965;2:197–200. [Google Scholar]
- 89.Galvão A.B., Silva e Souza H., Lima R.R. Triatoma williami n. sp. (Hemiptera, Triatominae) Rev. Bras. Mal. Doenças Trop. 1965;17:363–366. [PubMed] [Google Scholar]
- 90.Sherlock I.A., Serafim M. Triatoma lenti sp. n., Triatoma pessoai sp. n. e Triatoma bahiensis sp. n. do estado da Bahia, Brasil (Hemiptera, Reduviidae) Gaz. Méd. Bahia. 1967;67:75–92. (In Portuguese) [Google Scholar]
- 91.Galvão A.B., Souza H.A.S., Lima R.R. Especies de Triatominae ocorrentes em Goias e descrição de uma nova especie. Revista Brasileira de Malariologia e Doenças Tropicais. 1967;19:397–412. (In Portuguese) [PubMed] [Google Scholar]
- 92.Carcavallo R.U., Cichero J.A., Martínez A., Prosen A.F., Ronderos R. Una nueva espécie del género Triatoma Laporte (Hemiptera, Reduviidae, Triatominae) Segundas Jornadas Entomoepidemiológicas Argentinas. 1967;2:43–48. (In Spanish) [Google Scholar]
- 93.Galvão A.B., Palma J.D. Uma nova espécie do genero Panstrongylus Berg, 1879 (Reduviidae, Triatominae) Rev. Bras. Biol. 1968;28:403–405. (In Portuguese) [Google Scholar]
- 94.Zeledón R., Ponce C. Descripción de una nueva especie de Triatoma de Honduras, América Central (Hemiptera, Reduviidae) Rev. Biol. Trop. 1972;20:275–279. (In Spanish) [PubMed] [Google Scholar]
- 95.Almeida F.B., Santos E.I., Sposina G. Triatomíneos da Amazonia III. Acta Amaz. 1973;3:43–66. doi: 10.1590/1809-43921973032043. (In Portuguese) [DOI] [Google Scholar]
- 96.Ghauri M.S.K. The Indian triatomine genus Linshcosteus (Reduviidae) Syst. Ent. 1976;1:183–187. doi: 10.1111/j.1365-3113.1976.tb00037.x. [DOI] [Google Scholar]
- 97.Carcavallo R.U., Barreto P. Una nueva especie de Rhodnius Stål (Hemiptera, Reduviidae, Triatominae) de Colombia. Bol. Dir. Mal. San. Amb. 1976;16:176–183. (In Spanish) [Google Scholar]
- 98.Martínez A., Carcavallo R.U. Un nuevo Triatominae neotropical (Hemiptera: Reduviidae) Fol. Ent. Mex. 1977;38:109–118. (In Spanish) [Google Scholar]
- 99.Sherlock I.A., Guitton N., Miles M. Rhodnius paraensis espécie nova do Estado do Pará, Brasil (Hemiptera, Reduviidae, Triatominae) Acta Amaz. 1977;7:71–74. doi: 10.1590/1809-43921977071071. (In Portuguese) [DOI] [Google Scholar]
- 100.Else J.G., Cheong W.H., Mahadevan S., Zárate L.G. A new species of cave-inhabiting Triatoma (Hemiptera: Reduviidae) from Malaysia. J. Med. Ent. 1977;14:367–369. doi: 10.1093/jmedent/14.3.367. [DOI] [Google Scholar]
- 101.Lent H., Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas disease. Bull. Am. Mus. Nat. Hist. 1979;163:123–520. [Google Scholar]
- 102.Serra R.G., Atzingen N.C.B., Serra O.P. Nova espécie do gênero Alberprosenia Martínez & Carcavallo, 1977, do Estado do Pará, Brasil. V Congresso Bras. Par. 1980:126. (In Portuguese) [Google Scholar]
- 103.Martínez A., Carcavallo R.U., Pelaez D. Triatoma brailovskyi, nueva especie Triatominae de México. Chagas. 1984;1:39–42. (In Spanish) [Google Scholar]
- 104.Barrett T.V., Arias J.R. A new triatomine host of Trypanosoma cruzi from the Central Amazon of Brasil: Cavernicola lenti n. sp. (Hemiptera, Reduviidae, Triatominae) Memórias do Instituto Oswaldo Cruz. 1985;80:91–96. doi: 10.1590/S0074-02761985000100014. [DOI] [Google Scholar]
- 105.Carcavallo R.U., Martínez A., Peláez D. Una nueva especie de Triatoma Laporte, de México. Chagas. 1987;4:4–5. (In Spanish) [Google Scholar]
- 106.Fernandez-Loayza R. Triatoma matsunoi nueva especie del norte peruano (Hemiptera, Reduviidae, Triatominae) Rev. Per. Ent. 1989;31:21–24. (In Spanish) [Google Scholar]
- 107.Lent H., Jurberg J., Galvão C. Rhodnius stali n. sp., afim de Rhodnius pictipes Stål, 1872. Memórias do Instituto Oswaldo Cruz. 1993;88:605–614. doi: 10.1590/S0074-02761993000400019. [DOI] [Google Scholar]
- 108.Osuna E., Ayala J.M. Belminus pittieri, nueva especie de Bolboderini (Triatominae: Reduviidae: Heteroptera) Bol. Ent. Venez. 1993;8:147–150. (In Spanish) [Google Scholar]
- 109.Martínez A., Carcavallo R.U., Jurberg J. Triatoma gomeznunezi a new species of Triatomini from Mexico (Hemiptera, Reduviidae, Triatominae) Ent. Vect. 1994;1:15–19. [Google Scholar]
- 110.Lent H., Jurberg J., Carcavallo R.U. Belminus laportei sp. n. da região Amazônica (Hemiptera: Reduviidae: Triatominae) Memórias do Instituto Oswaldo Cruz. 1995;90:33–39. doi: 10.1590/S0074-02761995000100008. (In Portuguese) [DOI] [Google Scholar]
- 111.Frias D.A., Henry A.A., González C.R. Mepraia gajardoi: A new species of Triatominae (Hemiptera: Reduviidae) from Chile and its comparison with Mepraia spinolai. Rev. Chil. Hist. Nat. 1998;71:177–188. [Google Scholar]
- 112.Jurberg J., Rocha D.S., Lorosa E.S., Vinhaes M.C., Lent H. Uma nova espécie de Triatoma do Estado do Rio Grande do Sul, Brasil (Hemiptera, Reduviidae) Entom. Vect. 1998;5:295–310. (In Portuguese) [Google Scholar]
- 113.Carcavallo R.U., Galvão C., Lent H. Triatoma jurbergi sp. n. do norte do estado do Mato Grosso, Brasil (Hemiptera, Reduviidae, Triatominae) com uma atualização das sinonímias e outros táxons. Memórias do Instituto Oswaldo Cruz. 1998;93:459–464. doi: 10.1590/S0074-02761998000400007. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 114.Aguilar R.A., Torres B.N., Jímenez M.C., Jurberg J., Galvão C., Carcavallo R. Triatoma bassolsae sp. n. do México, com uma chave para as espécies do complexo “phyllosoma” (Hemiptera, Reduviidae) Memórias do Instituto Oswaldo Cruz. 1999;94:353–359. doi: 10.1590/S0074-02761999000300012. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 115.Mejia J.M., Galvão C., Jurberg J. Rhodnius colombiensis sp. n. da Colômbia, com quadros comparativos entre estruturas fálicas do gênero Rhodnius Stal, 1859 (Hemiptera, Reduviidae, Triatominae) Ent. Vect. 1999;6:601–617. (In Spanish) [Google Scholar]
- 116.Carcavallo R.U., Jurberg J. Triatoma baratai sp. n. do estado do Mato Grosso do Sul, Brasil (Hemiptera, Reduviidae, Triatominae) Ent. Vect. 2000;7:373–387. (In Portuguese) [Google Scholar]
- 117.Valente V.C., Valente S.A.S., Carcavallo R., Rocha D.S., Galvão C., Jurberg J. Considerações sobre uma nova espécie do gênero Rhodnius Stål, do estado do Pará, Brasil (Hemiptera, Reduviidae, Triatominae) Ent. Vect. 2001;8:65–80. (In Portuguese) [Google Scholar]
- 118.Carcavallo R.U., Jurberg J., Lent H., Galvão C., Steindel M., Pinto C.J.C. Nova especie do complexo oliveirai (nova denominação para o complexo matogrossensis) Hemiptera, Reduviidae, Triatominae) do estado do Rio Grande do Sul, Brasil. Memórias do Instituto Oswaldo Cruz. 2001;96:71–79. doi: 10.1590/S0074-02762001000100008. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 119.Galvão C., Pattersin J.S., Silva D.R., Jurberg J., Carcavallo R., Rajen K., Ambrose D.P., Miles M.A. A new species of triatomine from Tamil Nadul, India. Med. Vet. Ent. 2002;16:75–82. doi: 10.1046/j.0269-283x.2002.00351.x. [DOI] [PubMed] [Google Scholar]
- 120.Papa A.R., Jurberg J., Carcavallo R.U., Cerqueira R.L., Barata J.M.B. Triatoma sherlocki sp. n. coletada na Bahia, Brasil (Hemiptera, Reduviidae, Triatominae) Ent. Vect. 2002;9:133–146. (In Portuguese) [Google Scholar]
- 121.Carcavallo R.U., Jurberg J., Rocha D.S., Galvão C., Noireau F., Lent H. Triatoma vandae sp. n. do complexo oliveirai encontrada no Estado de Mato Grosso, Brasil (Hemiptera: Reduviidae: Triatominae) Memórias do Instituto Oswaldo Cruz. 2002;97:649–654. doi: 10.1590/S0074-02762002000500011. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 122.Poinar G., Jr. Triatoma dominicana sp. n. (Hemiptera: Reduviidae: Triatominae), and Trypanosoma antiquus sp. n. (Stercoraria: Trypanosomatidae), the first fossil evidence of a triatomine-trypanosomatid vector association. Vec. Borne. Zoon. Dis. 2005;5:72–81. doi: 10.1089/vbz.2005.5.72. [DOI] [PubMed] [Google Scholar]
- 123.Galvão C., Angulo V.M. Belminus corredori, a new species of Bolboderini (Hemiptera: Reduviidae: Triatominae) from Santander, Colombia) Zootaxa. 2006;1241:61–68. [Google Scholar]
- 124.Sandoval C.M., Pábon E., Jurberg J., Galvão C. Belminus ferroae n. sp. from the Colombian north-east, with a key to the species of the genus (Hemiptera: Reduviiade: Triatominae) Zootaxa. 2007;1443:55–64. doi: 10.11646/zootaxa.1443.1.5. [DOI] [Google Scholar]
- 125.Bérenger J.M., Blanchet D. A new species of the genus Panstrongylus from French Guiana (Heteroptera; Reduviidae; Triatominae) Memórias do Instituto Oswaldo Cruz. 2007;102:733–736. doi: 10.1590/S0074-02762007000600012. [DOI] [PubMed] [Google Scholar]
- 126.Martinez Avendaño E., Chávez Espada T., Sossa Gil D., Asturizaga R.A., Mamani B.V., Prieto P.V. Triatoma boliviana sp. n. (Hemiptera: Reduviidae: Triatominae) de los valles subandinos de La Paz-Bolivia, similar a Triatoma nigromaculata Stal, 1859. Bol. Inst. Invest. Salud. Desar. 2007;3:87–90. (In Spanish) [Google Scholar]
- 127.Costa J., Felix M. Triatoma juazeirensis sp. nov. from the state of Bahia, Northeastern Brazil (Hemiptera: Reduviidae: Triatominae) Memórias do Instituto Oswaldo Cruz. 2007;102:87–90. doi: 10.1590/S0074-02762007000100015. [DOI] [PubMed] [Google Scholar]
- 128.Ayala J.M. Una nueva especie de Panstrongylus Berg de Venezuela. (Hemiptera: Reduviidae, Triatominae) Entomotropica. 2009;24:105–109. (In Spanish) [Google Scholar]
- 129.Jurberg J., Rocha D.S., Galvão C. Rhodnius zeledoni sp. nov. afim de Rhodnius paraensis Sherlock, Guitton & Miles, 1977 (Hemiptera, Reduviidae, Triatominae) Biota Neotrop. 2009;9:123–128. [Google Scholar]
- 130.Poinar G., Jr. Panstrongylus hispaniolae sp. n. (Hemiptera: Reduviidae: Triatominae), a new fossil triatomine in Dominican amber, with evidence of gut flagellates. Palaeodiversity. 2013;6:1–8. [Google Scholar]
- 131.Gonçalves T.C.M., Teves-Neves S.C., Santos-Mallet J.R., Carbajal-de-la-Fuente A.L., Lopes C.M. Triatoma jatai sp. nov. in the state of Tocantins, Brazil (Hemiptera: Reduviidae: Triatominae) Memórias do Instituto Oswaldo Cruz. 2013;108:429–437. doi: 10.1590/S0074-0276108042013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oliveira J., Ayala J.M., Justi S.A., da Rosa J.A., Galvão C. Description of a new species of Nesotriatoma Usinger, 1944 from Cuba and revalidation of synonymy between Nesotriatoma bruneri (Usinger, 1944) and N. flavida (Neiva, 1911) (Hemiptera, Reduviidae, Triatominae) J. Vector. Ecol. 2018;43:148–157. doi: 10.1111/jvec.12294. [DOI] [PubMed] [Google Scholar]
- 133.Poinar G., Jr. A primitive triatomine bug, Paleotriatoma metaxytaxa gen. et sp. nov. (Hemiptera: Reduviidae: Triatominae), in mid-Cretaceous amber from northern Myanmar. Cret. Res. 2018;93:90–97. doi: 10.1016/j.cretres.2018.09.004. [DOI] [Google Scholar]
- 134.Monteiro F.A., Weirauch C., Felix M., Lazoski C., Abad-Franch F. Evolution, Systematics, and Biogeography of the Triatominae, Vectors of Chagas Disease. Adv Parasitol. 2018;99:265–344. doi: 10.1016/bs.apar.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 135.Castro M.R.J., Clément G., Monteiro F.A., Vieira C., Carareto C.M. Homology-free detection of transposable elements unveils their dynamics in three ecologically distinct Rhodnius species. Genes. 2020;11:170. doi: 10.3390/genes11020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Monteiro F.A., Barret T.V., Fitzpatrick S., Cordon-Rosales C., Feliciangeli D., Beard C.B. Molecular phylogeography of the Amazonian Chagas disease vectors Rhodnius prolixus and R. robustus. Mol. Ecol. 2003;12:997–1006. doi: 10.1046/j.1365-294X.2003.01802.x. [DOI] [PubMed] [Google Scholar]
- 137.Justi S.A., Russo C.A.M., Mallet J.R.S., Obara M.T., Galvão C. Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae) Parasites Vectors. 2014;7:149. doi: 10.1186/1756-3305-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Justi S.A., Galvão C., Schrago C.G. Geological Changes of the Americas and their Influence on the Diversification of the Neotropical Kissing Bugs (Hemiptera: Reduviidae: Triatominae) PLoS Negl. Trop. Dis. 2016;10:e0004527. doi: 10.1371/journal.pntd.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Panzera Y., Pita S., Ferreiro M.J., Ferrandis I., Lages C., Pérez R., Silva A.E., Guerra M., Panzera F. High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera) Cytog. Gen. Res. 2012;138:56–67. doi: 10.1159/000341888. [DOI] [PubMed] [Google Scholar]
- 140.Nascimento J.D., Caneguim B.H., Paula M.C., Ribeiro A.R., Sasso-Cerri E., Rosa J.A. Spermathecae: Morphofunctional features and correlation with fat bodies and trachea in six species of vectors of Chagas disease. Acta Trop. 2019;197:105032. doi: 10.1016/j.actatropica.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 141.Garcia B.A., Powell J.R. Phylogeny of species of Triatoma (Hemiptera: Reduviidae) based on mitochondrial DNA sequences. J. Med. Entomol. 1998;35:232–238. doi: 10.1093/jmedent/35.3.232. [DOI] [PubMed] [Google Scholar]
- 142.Hypša V., Tietz D., Zrzavy J., Rego R.O., Galvao C., Jurberg J. Phylogeny and biogeography of Triatominae (Hemiptera: Reduviidae): Molecular evidence of a New World origin of the Asiatic clade. Mol. Phylo. Evol. 2002;23:447–457. doi: 10.1016/S1055-7903(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 143.Lasserre D.F., Oliveira J., Pinotti H., Rosa J.A. Morphological description of Mepraia spp. females (Hemiptera: Reduviidae, Triatominae) Acta Trop. 2019;190:389–394. doi: 10.1016/j.actatropica.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 144.Rengifo-Correa L., Abad-Franch F., Martínez-Hernández F., Salazar-Schettino P.M., Téllez-Rendón J.L., Villalobos G., Morrone J.J. A biogeographic-ecological approach to disentangle reticulate evolution in the Triatoma phyllosoma species group (Heteroptera: Triatominae), vectors of Chagas disease. J. Zoolog. Syst. Evol. Res. 2020;59:94–110. doi: 10.1111/jzs.12409. [DOI] [Google Scholar]
- 145.Cesaretto N.R., Oliveira J., Ravazi A., Madeira F.F., Reis Y.V., Oliveira A.B.B., Vicente R.D., Cristal D.C., Galvão C., Azeredo-Oliveira M.T.V., et al. Trends in taxonomy of Triatomini (Hemiptera, Reduviidae, Triatominae): Reproductive compatibility reinforces the synonymization of Meccus Stål, 1859 with Triatoma Laporte, 1832. Parasit Vect. 2021;14:340. doi: 10.1186/s13071-021-04847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.International Commission on Zoological Nomenclature The International Code of Zoological Nomenclature. 1999. [(accessed on 28 October 2021)]. Available online: https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/the-code-online/
- 147.Pita S., Lorite P., Nattero J., Galvão C., Alevi K.C.C., Teves S.C., Azeredo-Oliveira M.T.V., Panzera F. New arrangements on several species subcomplexes of Triatoma genus based on the chromosomal position of ribosomal genes (Hemiptera-Triatominae) Infect. Genet. Evol. 2016;43:225–231. doi: 10.1016/j.meegid.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 148.Alevi K.C.C., Oliveira J., Azeredo-Oliveira M.T.V., Rosa J.A. Triatoma vitticeps subcomplex (Hemiptera, Reduviidae, Triatominae): A new grouping of Chagas disease vectors from South America. Parasites Vectors. 2017;10:180. doi: 10.1186/s13071-017-2129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mendonça V.J., Alevi K.C.C., Pinotti H., Gurgel-Gonçalves R., Pita S., Guerra A.L., Panzera F., Araújo R.F., Azeredo-Oliveira M.T.V., Rosa J.A. Revalidation of Triatoma bahiensis Sherlock & Serafim, 1967 (Hemiptera: Reduviidae) and phylogeny of the T. brasiliensis species complex. Zootaxa. 2016;4107:239–254. doi: 10.11646/zootaxa.4107.2.6. [DOI] [PubMed] [Google Scholar]
- 150.Oliveira J., Marcet P.L., Takiya D.M., Mendonça V.J., Belintani T., Bargues M.D., Mateo L., Chagas V., Folly-Ramos E., Cordeiro-Estrela P., et al. Combined phylogenetic and morphometric information to delimitand unify the Triatoma brasiliensis species complex and the Brasiliensis subcomplex. Act. Trop. 2017;170:140–148. doi: 10.1016/j.actatropica.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Belintani T., Oliveira J., Pinotti H., Silva L.A., Alevi K.C.C., Galvão C., Rosa J.A. Phylogenetic and phenotypic relationships of the Triatoma sordida subcomplex (Hemiptera: Reduviidae: Triatominae) Acta Trop. 2020;212:105679. doi: 10.1016/j.actatropica.2020.105679. [DOI] [PubMed] [Google Scholar]
- 152.Padial J.M., Miralles A., de la Riva I., Vences M. The integrative future of taxonomy. Front. Zool. 2010;7:16. doi: 10.1186/1742-9994-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dorn P.L., Calderon C., Melgar S., Moguel B., Solorzano E., Dumonteil E., Rodas A., Rua N., Garnica R., Monroy C. Two distinct Triatoma dimidiata (Latreille, 1811) taxa are found in sympatry in Guatemala and Mexico. PLoS Negl. Trop. Dis. 2009;3:e393. doi: 10.1371/journal.pntd.0000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dorn P.L., de la Rúa N.M., Axen H., Smith N., Richards B.R., Charabati J., Suarez J., Woods A., Pessoa R., Monroy C., et al. Hypothesis testing clarifies the systematics of the main Central American Chagas disease vector, Triatoma dimidiata (Latreille, 1811), across its geographic range. Infect. Genet. Evol. 2016;44:431–443. doi: 10.1016/j.meegid.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Panzera F., Hornos S., Pereira J., Cestau R., Canale D., Diotaiuti L., Dujardin J.P., Perez R. Genetic variability and geographic differentiation among three species of triatomine bugs (Hemiptera-Reduviidae) Am. J. Trop. Med. Hyg. 1997;57:732–739. doi: 10.4269/ajtmh.1997.57.732. [DOI] [PubMed] [Google Scholar]
- 156.Panzera F., Pita S., Nattero J., Panzera Y., Galvão C., Chavez T., de Arias A.R., Téllez L.C., Noireau F. Cryptic speciation in the Triatoma sordida subcomplex (Hemiptera, Reduviidae) revealed by chromosomal markers. Parasites Vectors. 2015;8:495–504. doi: 10.1186/s13071-015-1109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dias F.B.S., Jaramillo N., Diotaiuti L. Description and characterization of the melanic morphotype of Rhodnius nasutus Stål, 1859 (Hemiptera: Reduviidae: Triatominae) Rev. Soc. Bras. Med. Trop. 2014;47:637–641. doi: 10.1590/0037-8682-0007-2014. [DOI] [PubMed] [Google Scholar]
- 158.Pita S., Panzera F., Ferrandis I., Galvão C., Gómez-Palacio A., Panzera Y. Chromosomal divergence and evolutionary inferences in Rhodniini basead on the chromosomal location of ribosomal genes. Memórias do Instituto Oswaldo Cruz. 2013;108:376–382. doi: 10.1590/S0074-02762013000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mayr E. Animal Species and Evolution. Harvard University Press; Cambridge, MA, USA: 1963. [Google Scholar]
- 160.Mayr E. Populations, Species, and Evolution. Harvard University Press; Cambridge, MA, USA: 1970. [Google Scholar]
- 161.Dobzhansky T. Genetics of the Evolutionary Process. Columbia University Press; New York, NY, USA: 1970. [Google Scholar]
- 162.Calderón-Fernández G.M., Juárez M.P. The cuticular hydrocarbons of the Triatoma sordida species subcomplex (Hemiptera: Reduviidae) Memórias do Instituto Oswaldo Cruz. 2013;108:778–784. doi: 10.1590/0074-0276108062013015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nattero J., Piccinali R.M., Lopes C.M., Hernandez M.L., Abrahan L., Lobbia P.A., Rodríguez C.S., Carbajal de la Fuente A.L. Morphometric variability among the species of the Sordida subcomplex (Hemiptera: Reduviidae: Triatominae): Evidence for differentiation across the distribution range of Triatoma sordida. Parasit. Vectors. 2017;10:412. doi: 10.1186/s13071-017-2350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oscherov E.B., Damborsky M.P., Bar M.E. Características biológicas de Triatoma sordida (Heteroptera, Reduviidae): Ciclo de vida. Revista de la Sociedad Entomológica Argentina. 1998;57:13–17. (In Spanish) [Google Scholar]
- 165.Souza J.M.P., Rodrigues V.L.C.C., Rocha e Silva E.O. Triatoma sordida: Considerações sobre o tempo de vida das formas adultas e sobre a oviposição das fêmeas. Revista de Saúde Pública. 1978;12:291–296. doi: 10.1590/S0034-89101978000300004. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 166.Pinto C.F. Fatos curiosos sobre a biologia do Triatoma sordida (Nota prévia) Rev. Soc. Bras. Med. 1949;6:305. (In Spanish) [Google Scholar]
- 167.Rosa J.A., Souza E.S., Costa T.A., Barbosa R.R., Souza A.J., Belintani T., Nascimento J.D., Gil-Santana H.R., Oliveira J. Third record of Rhodnius amazonicus and comparative study with R. pictipes (Hemiptera, Reduviidae, Triatominae) Acta Trop. 2017;176:364–372. doi: 10.1016/j.actatropica.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 168.Rodrigues J.M.S., Rosa J.A., Moreira F.F.F., Galvão C. Morphology of the terminal abdominal segments in females of Triatominae (Insecta: Hemiptera: Reduviidae) Acta Trop. 2018;185:86–97. doi: 10.1016/j.actatropica.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 169.Dale C., Almeida C.E., Mendonça V.J., Oliveira J., Rosa J.A., Galvão C., Costa J. An updated and illustrated dichotomous key for the Chagas disease vectors of Triatoma brasiliensis species complex and their epidemiologic importance. Zookeys. 2018;805:33–43. doi: 10.3897/zookeys.805.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Osório-Quintero L., Ceretti W., Vendramini D.P., Rosa J.A., Oliveira J., Obara M.T., Barata J.M.S. Morphological study of the urotergite I process in ten species of the genus Triatoma (Hemiptera, Reduviidae, Triatominae) Acta Trop. 2019;192:112–122. doi: 10.1016/j.actatropica.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 171.Almeida M.A.R.C., Freitas S.P.C., Oliveira L.R., Lima N.R.C., Rangel E.F., Santos-Mallet J. Characterization of the Buccula, Rostrum, Stridulatory Sulcus, Scutellum, and External Female Genitalia of Triatoma carcavalloi (Jurberg, Rocha & Lent, 1998), Triatoma circummaculata (Stål, 1859), and Triatoma rubrovaria (Blanchard, 1843) (Hemiptera, Reduviidae, Triatominae) J. Parasitol. Res. 2019;2019:3517098. doi: 10.1155/2019/3517098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oliveira J., Almeida C.E., Mendonça V.J., Alevi K.C.C., Costa J., Rosa J.A. Triatoma brasiliensis species complex: Characterization of the external female genitalia. J. Vector Ecol. 2020;45:57–68. doi: 10.1111/jvec.12373. [DOI] [PubMed] [Google Scholar]
- 173.Gurgel-Gonçalves R., Komp E., Campbell L.P., Khalighifar A., Mellenbruch J., Mendonça V.J., Owens H.L., Felix K.C., Peterson A.T., Ramsey J.M. Automated identifcation of insect vectors of Chagas disease in Brazil and Mexico: The Virtual Vector Lab. PeerJ. 2017;5:e3040. doi: 10.7717/peerj.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khalighifar A., Komp E., Ramsey J.M., Gurgel-Gonçalves R., Peterson A.T. Deep learning algorithms improve automated identifcation of Chagas disease vectors. J. Med. Entomol. 2019;56:1404–1410. doi: 10.1093/jme/tjz065. [DOI] [PubMed] [Google Scholar]
- 175.Actis A.S., Traversa O.C., Carcavalho R.U. Estudios taxonómicos sobre el genero Triatoma Laporte mediante la electrophoresis de la linfa. An. Esc. Nac. Cienc. Biol. 1964;3:97–106. (In Spanish) [Google Scholar]
- 176.Dujardin J.P., Chávez T., Moreno J.M., Machane M., Noireau F., Schofield C.J. Comparison of isoenzyme electrophoresis and morphometric analysis for phylogenetic reconstruction of the Rhodniini (Hemiptera: Reduviidae: Triatominae) J. Med. Entomol. 1999;36:653–659. doi: 10.1093/jmedent/36.6.653. [DOI] [PubMed] [Google Scholar]
- 177.Costa J., Freitas-Sibajev M.G.R., Marchon-Silva V., Pires M.Q., Pacheco R.S. Isoenzymes detect variation in populations of Triatoma brasiliensis (Hemiptera, Reduviidae, Triatominae) Memórias do Instituto Oswaldo Cruz. 1997;92:459–464. doi: 10.1590/S0074-02761997000400002. [DOI] [PubMed] [Google Scholar]
- 178.Flores A., Magallón-Gastélum E., Bosseno M.F., Ordóñez R., Lozano-Kasten F., Espinoza B., Ramsey J., Breniére S.F. Isoenzyme variability of five principal triatomine vector species of Chagas disease in Mexico. Infect. Genet. Evol. 2001;1:21–28. doi: 10.1016/S1567-1348(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 179.Ueshima N. Cytotaxonomy of The Triatominae (Reduviidae: Hemiptera) Chromosoma. 1966;18:97–122. doi: 10.1007/BF00326447. [DOI] [Google Scholar]
- 180.Alevi K.C.C., Mendonça P.P., Pereira N.P., Rosa J.A., Azeredo-Oliveira M.T.V. Karyotype of Triatoma melanocephala Neiva and Pinto (1923). Does this species fit in the Brasiliensis subcomplex? Infect. Genet. Evol. 2012;12:1652–1653. doi: 10.1016/j.meegid.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 181.Alevi K.C.C., Borsatto K.C., Moreira F.F.F., Jurberg J., Azeredo-Oliveira M.T.V. Karyosystematics of Triatoma rubrofasciata (De Geer, 1773) (Hemiptera: Reduviidae: Triatominae) Zootaxa. 2015;3994:433–438. doi: 10.11646/zootaxa.3994.3.7. [DOI] [PubMed] [Google Scholar]
- 182.Alevi K.C.C., Borsatto K.C., Moreira F.F.F., Jurberg J., Azeredo-Oliveira M.T.V. Karyosystematic and karyotype evolution of Panstrongylus lutzi (Neiva & Pinto, 1923) (Hemiptera, Triatominae) Braz. J. Biol. 2017;78:180–182. doi: 10.1590/1519-6984.166442. [DOI] [PubMed] [Google Scholar]
- 183.Alevi K.C.C., Oliveira J., Rosa J.A., Azeredo-Oliveira M.T.V. Karyotype Evolution of Chagas Disease Vectors (Hemiptera, Triatominae) Am. J. Trop. Med. Hyg. 2018;99:87–89. doi: 10.4269/ajtmh.17-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Panzera F., Pérez R., Panzera Y., Ferrandis I., Ferreiro M.J., Calleros L. Cytogenetics and Genome Evolution in the Subfamily Triatominae (Hemiptera, Reduviidae). Cytogen. Gen. Res. 2010;128:77–87. doi: 10.1159/000298824. [DOI] [PubMed] [Google Scholar]
- 185.Imperador C.H.L., Moreira F.F.F., Rosa J.A., Azeredo-Oliveira M.T.V., Alevi K.C.C. Cytotaxonomy of the Maculata subcomplex (Hemiptera, Triatominae) Braz. J. Biol. 2017;77:887–889. doi: 10.1590/1519-6984.20915. [DOI] [PubMed] [Google Scholar]
- 186.Bardella V.B., Pita S., Vanzela A.L.L., Galvão C., Panzera F. Heterochromatin base pair composition and diversification in holocentric chromosomes of kissing bugs (Hemiptera, Reduviidae) Memórias do Instituto Oswaldo Cruz. 2016;111:614–662. doi: 10.1590/0074-02760160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alevi K.C.C., Bittinelli I.F., Delgado L.M.G., Madeira F.F., Oliveira J., Lilioso M., Folly-Ramos E., Rosa J.A., Azeredo-Oliveira M.T.V. Molecular cytotaxonomy of the Triatoma brasiliensis species subcomplex (Hemiptera, Triatominae) Acta Trop. 2020;201:105225. doi: 10.1016/j.actatropica.2019.105225. [DOI] [PubMed] [Google Scholar]
- 188.Ravazi A., Oliveira J., Campos F.F., Madeira F.F., Reis Y.V., Oliveira A.B.B., Azeredo-Oliveira M.T.V., Rosa J.A., Galvão C., Alevi K.C.C. Trends in evolution of the Rhodniini tribe (Hemiptera, Triatominae): Experimental crosses between Psammolestes tertius Lent & Jurberg, 1965 and P. coreodes Bergroth, 1911 and analysis of the reproductive isolating mechanisms. Parasites Vectors. 2021;14:350. doi: 10.1186/s13071-021-04854-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pita S., Lorite P., Cuadrado A., Panzera Y., Oliveira J., Alevi K.C.C., Rosa J.A., Freitas S.P.C., Gómez-Palacio A., Solari A., et al. High chromosomal mobility of ribosomal clusters in holocentric chromosomes of Triatominae, vectors of Chagas disease (Hemiptera-Reduviidae) Med. Vet. Entomol. 2021 doi: 10.1111/mve.12552. In press. [DOI] [PubMed] [Google Scholar]
- 190.Borsatto K.C., Azeredo-Oliveira M.T.V., Alevi K.C.C. Identification Key for the Chagas Disease Vectors of Five Brazilian States, Based on Cytogenetic Data. Am. J. Trop. Med. Hyg. 2019;100:303–305. doi: 10.4269/ajtmh.18-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Borsatto K.C., Reis Y.V., Garcia A.C.C., Sousa P.S., Azeredo-Oliveira M.T.V., Alevi K.C.C. CytoKey: Identification Key for the Chagas Disease Vectors of the Largest Brazilian Urban Center (São Paulo State), Based on Cytogenetic Data. Am. J. Trop. Med. Hyg. 2019;101:113–115. doi: 10.4269/ajtmh.18-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Oliveira J., Rosa J.A., Alevi K.C.C. Chagas Disease Vectors of Espírito Santo, Brazil: First Report of Triatoma infestans (Klug, 1834) (Hemiptera, Triatominae) in the Brazilian State and Development of an Identification Key Based on Cytogenetic Data. Am. J. Trop. Med. Hyg. 2021;104:653–655. doi: 10.4269/ajtmh.20-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gonzalez-Britz N.E.G., Alevi K.C.C., Garcia A.C., Martínez-Purroy C.E., Galvão C., Carrasco H.J. Chagas disease vectors of Paraguay: Entomoepidemiological aspects of Triatoma sordida (Stål, 1859) and development of an identification key for Paraguayan triatomines based on cytogenetics data. Am. J. Trop Med. Hyg. 2021;105:130–133. doi: 10.4269/ajtmh.20-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laroche M., Bérenger J.M., Gazelle G., Blanchet D., Raoult D., Parola P. MALDI-TOF MS protein profiling for the rapid identification of Chagas disease triatomine vectors and application to the triatomine fauna of French Guiana. Parasitology. 2018;145:665–675. doi: 10.1017/S0031182017001342. [DOI] [PubMed] [Google Scholar]
- 195.Souza E.S., Fernandes R.P., Guedes W.N., Santos F.N., Eberlin M.N., Lopes N.P., Padovani V.D., Rosa J.A. Rhodnius spp. are differentiated based on the peptide/protein profile by matrix-assisted laser desorption/ionization mass spectrometry and chemometric tools. Anal. Bioanal. Chem. 2020;412:1431–1439. doi: 10.1007/s00216-019-02376-y. [DOI] [PubMed] [Google Scholar]
- 196.Souza É.S., Fernandes R.P., Galvão C., Paiva V.F., Rosa J.A. Distinguishing two species of Cavernicola (Hemiptera, Reduviidae, Triatominae) with matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Acta Trop. 2019;98:105071. doi: 10.1016/j.actatropica.2019.105071. [DOI] [PubMed] [Google Scholar]
- 197.Carvalho D.B., Congrains C., Chahad-Ehlers S., Pinotti H., De Brito R.A., Da Rosa J.A. Differential transcriptome analysis supports Rhodnius montenegrensis and Rhodnius robustus (Hemiptera, Reduviidae, Triatominae) as distinct species. PLoS ONE. 2017;12:e0174997. doi: 10.1371/journal.pone.0174997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brito R.N., Geraldo J.A., Monteiro F.A., Lazoski C., Souza R.C.M., Abad-Franch F. Transcriptome-based molecular systematics: Rhodnius montenegrensis (Triatominae) and its position within the Rhodnius prolixus-Rhodnius robustus cryptic-species complex. Parasit. Vectors. 2019;12:305. doi: 10.1186/s13071-019-3558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.