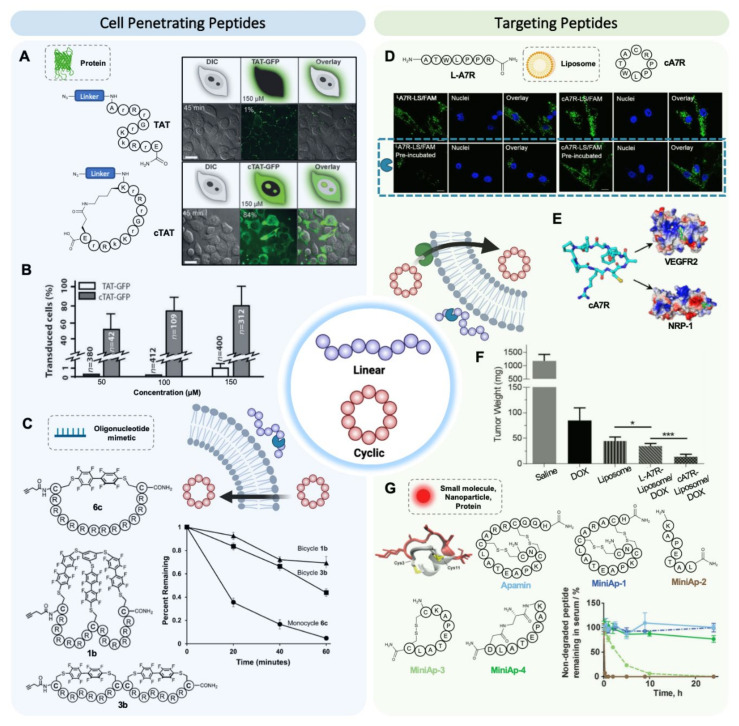
Figure 3.
Cyclization of peptides enhances proteolytic stability and thus improves cell penetration or targeting. (A) Chemoselective conjugation via azide–alkyne cycloaddition of GFP to cTAT enhances transduction of living cells. Confocal microscopy images (scale bar = 15 µm) show efficient transduction of 84% of HeLa cells with the cTAT–GFP conjugate, compared with the 1% achieved by TAT–GFP, adapted with permission from [44], John Wiley & Sons, 2014. (B) Quantification of the percentage of transduced cells for 50, 100, and 150 µM of TAT–GFP and cTAT–GFP [44]. (C) Bicyclic peptides demonstrate enhanced proteolytic stability relative to monocyclic peptides after incubation with trypsin at 37 °C for up to 1 h, adapted with permission from [46], John Wiley & Sons, 2018. (D) Cyclic A7R-conjugated liposomes (LS) retain binding capacity to U87 cells after pre-incubation with 50% mouse serum for 4 h, while binding of L-A7R-LS is dramatically reduced, adapted with permission from [50], Elsevier, 2015. (E) 3D model of cA7R and its binding mode on VEGFR2 and NRP-1 receptors, as simulated by molecular docking [50]. (F) cA7R-conjugated LS loaded with doxorubicin (DOX) shows a very significant reduction in U87-derived xenograft weight in nude mice, confirming the positive influence of the cyclic structure on therapeutic efficacy [50]. (G) 3D representation of MiniAp-4. Proteolytic stability of the BBB shuttle apamin and its four derivatives MiniAp1–4 after incubation with 90% human serum at 37 °C for 24 h, adapted with permission from [52], John Wiley & Sons, 2015.