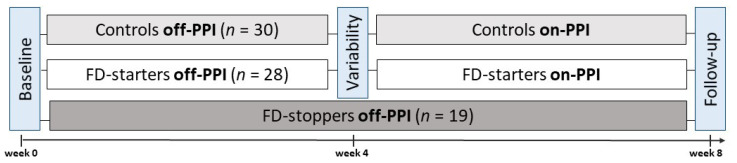
Figure 1.
Study design and procedures. Procedures were performed at baseline, after 2–4 weeks (variability) and after 4 weeks of Pantoprazole 40 mg once daily (follow-up) in controls and FD-starters. For FD-stoppers, procedures were performed at baseline and after 8 weeks of PPI withdrawal (off-PPI).