Abstract
Pseudomonas aeruginosa is an opportunistic, Gram-negative pathogen and an important cause of hospital acquired infections, especially in immunocompromised patients. Highly virulent P. aeruginosa strains use a type III secretion system (T3SS) to inject exoenzyme effectors directly into the cytoplasm of a target host cell. P. aeruginosa strains that express the T3SS effector, ExoU, associate with adverse outcomes in critically ill patients with pneumonia, owing to the ability of ExoU to rapidly damage host cell membranes and subvert the innate immune response to infection. Herein, we review the structure, function, regulation, and virulence characteristics of the T3SS effector ExoU, a highly cytotoxic phospholipase A2 enzyme.
Keywords: Pseudomonas aeruginosa, ExoU, phospholipase A2, pneumonia, innate immunity, amyloids, inflammasomes
1. Introduction
Pseudomonas aeruginosa is a Gram-negative, rod-shaped bacterium found in soil and water. Similar to other pseudomonads and mucosal pathogens, P. aeruginosa possesses a single polar flagellum for motility [1]. Due to its biosynthetic capacity, P. aeruginosa thrives in environments with minimal nutrients and under diverse physical conditions, allowing persistence in hospital and community settings [2]. As an important cause of nosocomial pneumonia globally, P. aeruginosa is classified as an ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogen listed as critical priority for new research and drug development by the World Health Organization [3,4,5]. Healthcare infections associated with P. aeruginosa exceed 51,000 cases annually in the United States and are responsible for 40% of deaths of patients with ventilator-associated pneumonia (VAP) [6,7,8,9,10]. P. aeruginosa causes both acute and chronic infections. Severe burn, mechanically ventilated, and immunocompromised patients are particularly susceptible to acute P. aeruginosa infections [11,12]. Patients with pre-existing lung disease, such as cystic fibrosis, are susceptible to chronic P. aeruginosa infections [11,12].
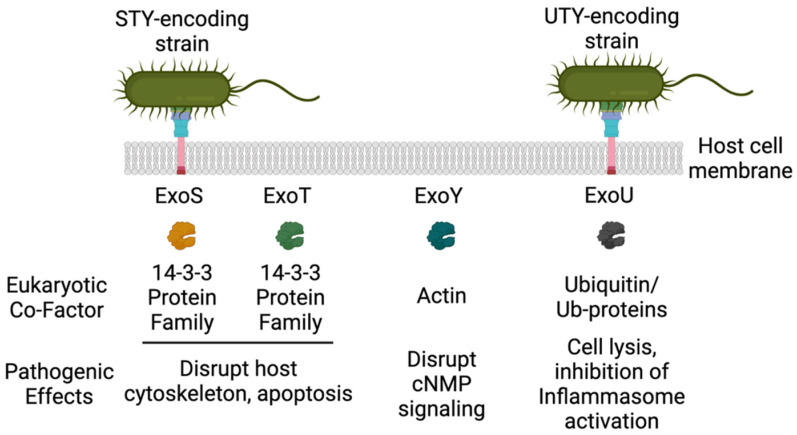
P. aeruginosa is a pathogen commonly isolated from patients with acute respiratory infections, which result from airway epithelial damage due to mechanical ventilation [13,14]. Despite improvements in mechanical ventilation strategies, such as reduced tidal volumes, patients often suffer injury to their innate lung barriers due to the endotracheal tubing, allowing the pathogen to attach and colonize mucous membranes [13,15]. Epithelial and alveolar macrophages become activated and damaged upon P. aeruginosa colonization of the airways [16,17]. Neutrophils and phagocytic-mediated clearance mechanisms are also impaired by a diverse array of pathogen-encoded virulence factors [18,19]. P. aeruginosa uses a wide array of virulence mechanisms to cause disease in humans (for excellent reviews see [20,21]). The P. aeruginosa type III secretion system (T3SS) is one of the most notorious of these virulence mechanisms, and aids in colonization of the airway and in immune avoidance [22]. To date, there are at least four well-characterized effector proteins referred to as Exoenzymes: ExoU, S, T, and Y [13]. Once delivered into the host cell cytoplasm, these effector proteins become activated through interactions with their cognate eukaryotic cofactors (Figure 1). Interestingly, P. aeruginosa clinical and environment strains vary in the cadre of T3 effectors encoded, which defines the pathophysiology of a given strain/isolate [8,23,24]. The relative distribution of T3 effectors is: ~35% ExoU, ~65% ExoS, ~100% ExoT, and ~90% ExoY [25]. ExoS and ExoT were the first P. aeruginosa T3 effectors identified and are bifunctional enzymes possessing GTPase activating protein (GAP) and ADP-ribosyltransferase activities that trigger pathological rearrangements in the host cell cytoskeleton [26]. These activities lead to cytotoxicity (ExoS) or host cell cytoskeletal rearrangements that limit phagocytosis (ExoT). Intoxication of cells by ExoS is characterized by cell death that takes several hours to manifest and results ultimately in apoptosis [27]. ExoY is a promiscuous nucleotidylyl cyclase [28,29] that disrupts inter-endothelial gap junctions and promotes hyperphosphorylation and release of tau and amyloid-β [30,31,32,33]. ExoU was identified based on the observation that several clinical isolates possessed a rapid or acute cytotoxic response when co-cultivated with epithelial cells, which was distinct from the pattern of cell death induced by ExoS [34]. P. aeruginosa ExoU has been associated with severe disease outcomes in humans and in animal infection models [10,35]. The goal of this review is to provide an update on the role of ExoU in pathogenesis in the context of structure, function, and virulence relationships.
Figure 1.
The P. aeruginosa T3SS effectors, their activators, and host cell effects (Created with BioRender.com).
2. ExoU
Of the four effector proteins, ExoU is the most acutely cytotoxic. ExoU was identified in 1997 as an effector secreted by P. aeruginosa strain PA103 [34,36]. The gene encoding ExoU was identified as part of a mutant library screen and verified by peptide sequence analyses. ExoU is a 74-kDa (686 amino acid) protein that has a 5-residue amino-terminal sequence similar to ExoS and ExoT, which may be important for targeting to the T3SS [34]. Unlike the other effectors, exoU and its chaperone, spcU, are thought to have been acquired through horizontal gene transfer and reside on an 81-kb pathogenicity island [37]. In support of this hypothesis, yeast recombinational cloning and sequence analysis revealed exoU has a lower G + C content (59%) compared to exoS encoded by strain PAO1 (67%) and flanking elements homologous to IS407 [37]. In epithelial cells, ExoU expression is associated with rapid cellular lysis that occurs within 3 h of co-culture with P. aeruginosa. Cytotoxicity was so rapid that to mechanistically understand how ExoU functioned intracellularly, a system using Saccharomyces cerevisiae as a surrogate expression host was developed. ExoU synthesis could be controlled in yeast experimental model and the yeast cell wall protected the cell long enough to facilitate visualization of ExoU-induced damage to intracellular membranes and vacuolar fragmentation [38]. The addition of phospholipase inhibitors such as methyl arachidonyl fluorophosphonate (MAFP) significantly decreased ExoU cytotoxicity in yeast and mammalian cells. Combined with the nucleotide alignment analyses revealing a PLA domain similar to patatin, cPLA2, and iPLA2 [38], ExoU was postulated to function as a phospholipase hydrolyzing neutral lipids and phospholipids [38,39,40]. This model reconciled the rapid cytotoxicity in vitro and significant tissue destruction mediated by ExoU producing strains in animal models of infection.
2.1. Structure-Activity Relationships of ExoU
ExoU is a relativity large protein (686 amino acids, 74 kDa) making domain mapping somewhat complex. It was known that the protein likely possessed multiple functional domains even before crystal structures were available. Early studies of truncated proteins transfected into Chinese Hamster Ovary cells suggested that N- and C-terminal regions were necessary but not sufficient for toxicity [41]. Sato et al. and Philips et al. independently identified and verified, by site-specific mutagenesis studies, that ExoU was, indeed, part of the patatin family of phospholipases [38,39]. Patatin is a major storage protein in potatoes and other tubers that hydrolyzes phospholipids, p-nitro-phenyl esters, and monoacylglycerols [40,42]. Environmental stress or pathogenic infection results in patatin enzyme activation [40,42]. The patatin family of enzymes share functional similarity to eukaryotic cPLA2 and iPLA2, and possess PLA2 activity that hydrolyzes the acyl group at the sn-2 position of phospholipids, resulting in release of free fatty acids, such as lysophospholipids and arachidonic acid. There are three major conserved regions between ExoU, patatin, cPLA2, and iPLA2: a glycine-rich motif (oxyanion hole, G-G-X-R/K), a hydroxylase motif with a catalytic serine (G-X-S-X-G) and a catalytic aspartate motif (D-X-G/A). The latter two are commonly referred to as a serine-aspartate catalytic dyad, S142, and D344 for ExoU [38,43,44]. The catalytic aspartate is postulated to remove a proton from the catalytic serine, which allows the serine nucleophile to attack the carbonyl group of the ester located in the sn-2 position on the sn-glycerol-3-phosphate backbone of the phospholipid. The glycine-rich motif stabilizes the transition state. This stabilized state precedes collapse of the tetrahedral intermediate and release of lysophospholipid, leaving a serine-acyl intermediate. A similar mechanism is thought to apply to the hydrolysis of the serine-acyl intermediate. In this case, the catalytic aspartate activates water for a nucleophilic attack of the carbonyl carbon of the ester. Alanine substitutions at S142 or D334 render ExoU non-toxic and unable to cleave phospholipids. These and earlier studies identifying the binding domain for the ExoU chaperone, SpcU [45], indicated that the amino-terminal half of ExoU was required for T3SS secretion and contained the catalytic domain.
Functional mapping studies have identified several important regions within the ExoU C-terminus and downstream of the catalytic domain (positions past D344). Rabin et al. utilized peptide fusions to green fluorescent protein to map a plasma membrane localization domain (MLD) that resides between residues 550–687 [46]. Analysis of linker-insertions suggested that C-terminal residues were somehow associated with recognition of the eukaryotic co-factor protein required for activity [47]. Error-prone mutagenesis studies identified specific amino acid residues I609, Q623, N627, I654, R661, and A678 that appeared to play roles in both membrane localization and catalysis. Additionally, these studies indicated that a non-proteinaceous co-factor may contribute to ExoU phospholipase activity [48]. Stirling et al. demonstrated that a small C-terminal region (679–683) controls both membrane localization and the addition of diubiquitin at lysine 178 [49]. Covalent modification of ExoU was not required for toxicity, however. Overall, the C-terminal region of ExoU is important for both activity and the co-factor interactions that are postulated to facilitate conformational changes from an apoenzyme (inactive) to a holoenzyme (active) state [50].
2.2. Crystal Structure of ExoU
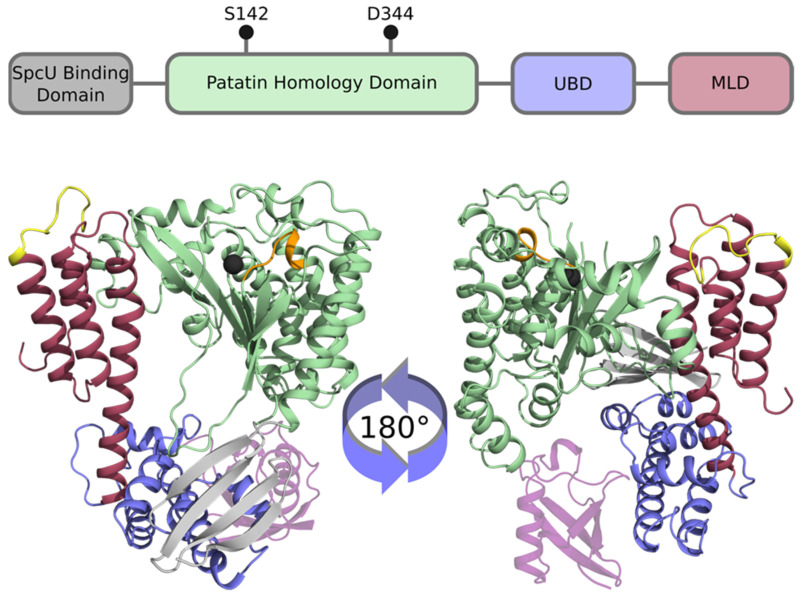
The functional mapping data and domain structure of ExoU was resolved when two independent groups published crystal structures of ExoU in complex with its chaperone, SpcU [51,52]. The structures are in good agreement with each other and show a complex multidomain organization. Figure 2 shows a structural diagram and ribbon models of ExoU based on the published structural solution data discussed herein. Domain 1 consists of a SpcU chaperone-binding motif located at the N-terminus comprising residues 55–101 [52]. Binding of SpcU to ExoU keeps the protein in an inactive state in the bacterial cytosol [52]. In addition, SpcU guides ExoU to the T3SS where it unfolds and is secreted through the injectisome into the host cell cytosol [53]. Following the chaperone-binding domain is the patatin-like domain which encodes the PLA2, residues 106–471. Within this region is the serine-aspartate catalytic dyad that is required for ExoU cytotoxicity [52]. At the C-terminus is a bridging domain, residues 480–580, and a membrane localization domain (MLD) comprising domains 3 and 4, residues 588–687 [51,52]. The MLD is a 4-helix bundle connected by several loop regions. Approximately 25% of the amino acid residues cannot be localized from the electron density map, including the catalytic aspartate at position 344, suggesting a fair amount of structural flexibility.
Figure 2.
The domain structure of ExoU. (Top) Cartoon diagram of the domain structure of ExoU. The N-terminal SpcU binding domain is shown in gray, followed by the catalytic patatin homology domain. Black pins indicate the approximate location of the catalytic dyad, S142 and D344. The ubiquitin-binding domain (UBD, bridging domain) is shown in blue followed by the membrane localization domain (MLD) in red. (Bottom) ExoU structural model. Functional domains are highlighted using the same colors as A with the addition of ubiquitin (transparent purple). The catalytic serine is marked as a black sphere and the catalytic aspartate is not modeled. Adjacent to the sphere is the conserved G-G-X-R/K motif shown in orange. The membrane interacting loop 3 of the C-terminal 4-helix bundle is highlighted in yellow. Loops missing from the crystal structures were modeled as previously described [54].
2.3. ExoU PLA2 Activity and Regulation by Eukaryotic Co-factors
Eukaryotic cell models and in vitro biochemistry techniques have been useful tools to characterize the regulation of ExoU PLA2 activation. Cloning and expression of ExoU as a recombinant protein revealed that the predicted PLA2 activity was readily detectable in vivo and in cell culture models. However, in vitro assays showed that the predicted PLA2 activity was only detectable when extracts of a eukaryotic cell (e.g., mammalian cells or yeast) were added to reactions containing phospholipid substrates. These experiments suggested that a eukaryotic factor was needed for ExoU activation, a regulatory feature that has also been described for ExoS, ExoT, and ExoY. To identify the eukaryotic activator of ExoU, Sato et al. performed biochemical enrichment experiments using cell extracts and selecting fractions that activated ExoU. These studies led to the discovery that SOD1 from either bovine or yeast sources activated ExoU in a dose-dependent manner [55]. However, SOD1 was not saturable in kinetic assays, suggesting that only part of the enriched fraction could activate ExoU [56,57]. Further analyses demonstrated that activation of ExoU by SOD1 was due a small subpopulation of molecules that was modified or contaminated with ubiquitin. Anderson et al. demonstrated that ubiquitin itself is a potent activator of ExoU PLA2 activity. Polyubiquitin and ubiquitinylated proteins also activate ExoU [57]. Ubiquitin is an abundant protein in all eukaryotic cells (up to 5% of total protein) but is absent in prokaryotes [58], making it an ideal activator of a protein with lethal activity. With crystal structures in hand, Tessmer et al. used computational, biophysical and biochemical approaches to map the ubiquitin interface to alpha helix 18 of ExoU and the hydrophobic face of ubiquitin [59,60]. Key residues of ExoU include T519, T522, V523, and S527 within the bridging domain [61].
Phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] has also been identified as both an activator and substrate for ExoU [51,62,63,64]. PI(4,5)P2 is also only present in eukaryotes and makes up approximately 1% of the total phospholipid content [65]. PI(4,5)P2 is associated with the inner leaflet of the cellular plasma membrane and participates in a variety of membrane-associated functions including motility, trafficking, signaling, and phagocytosis [65]. ExoU binds to liposomes containing 0.5% PI(4,5)P2 with an affinity of approximately 182 µM whereas liposomes lacking PI(4,5)P2 displayed a dissociation constant of 1.1 mM [66]. A PI(4,5)P2 binding motif is located within loop 3 of the C-terminal 4 helix bundle. Residue R661 serves as a key positively charged amino acid that interacts the negatively charged phosphate moieties of PI(4,5)P2 [62]. Using site-directed spin-labeling electron paramagnetic resonance spectroscopy, sites consisting of a basic-hydrophobic motif (residues 660–670, ARGFLRFGKPL) were found to be motionally dynamic when ExoU was analyzed with buffer alone (apo state), but transitioned to a more restricted state when ExoU was incubated with liposomes and diubiquitin (holo state, [60]). Accessibility to NiEDDA relaxation reagents further suggest that the loop inserts into the phospholipid bilayer, effectively anchoring ExoU to substrate [60]. PI(4,5)P2 may also serve to oligomerize ExoU to mediate optimal enzymatic activity [64]. In terms of regulating biochemical function, high affinity binding of ExoU to PI(4,5)P2 is important for plasma membrane targeting and maximal enzyme activity. Biologically, this interaction is likely linked to cleavage, disassembly of membrane focal adhesion complexes and cytoskeletal collapse associated with the early stages of ExoU intoxication [67].
Structural biology and biochemical studies of ExoU activation serve as a scaffold for the rational design of inhibitors aimed at reducing ExoU-mediated cytotoxicity in cells. Strategies to develop novel therapeutics are critical to combating infections with P. aeruginosa; a multi-drug resistant ESKAPE pathogen. Lee and colleagues utilized a high-throughput cell-based assay to screen chemical compounds and identified an ExoU PLA2 activity specific inhibitor named Pseudolipasin A [68]. Pseudolipasin A (PsA) did not affect bacterial growth or ExoU secretion through the T3SS, but it specifically targeted the enzymatic activity of ExoU, reducing the virulence of P. aeruginosa strains encoding ExoU. Treatment with PsA provided yeast, amoeba, and mammalian cells protection from ExoU-mediated killing [68]. It will be important for future studies to test PsA in pre-clinical animal infection models to determine any potential therapeutic utility in vivo. In 2014, a group of researchers identified a small series of arylsulfonamide compounds that inhibit ExoU cytotoxicity in a yeast-based screening assay [69]. S. cerevisiae was transformed with an ExoU expressing plasmid regulated by a copper-inducible expression system (pDH105). Cell viability measured 48 h post treatment with 5 µM of the different compounds identified arylsulfonamide [69]. Coupling amines with sulfonyl chlorides produced several compounds that inhibited ExoU-mediated cytotoxicity in yeast and mammalian cells with inhibitory activity similar to PsA [69]. Whether these arylsulfonamide compounds inhibit ExoU PLA2 activity, cofactor binding, or alter ExoU conformational state remains to be determined [70]. Furthermore, two potential ExoU inhibitors have been identified, PsA and arylsulfonamide; in-depth analyses of structure-activity relationships are pertinent to further characterize these inhibitors or to generate new inhibitors targeting ExoU.
2.4. ExoU Orthologues
Ubiquitin-mediated activation of patatin-like enzymes was postulated to be a conserved process in bacteria. Anderson et al. performed an in silico bioinformatics survey of bacterial genomic sequences to identify patatin-like enzymes [66]. The number of candidates were narrowed with additional search criteria that included possession of a T3 or T4SS for cytoplasmic delivery of the enzyme and a size exceeding 500 residues. Enzymes from bacteria with different lifestyles were purified, tested biochemically, or analyzed in prokaryotic and eukaryotic toxicity assays. Common properties included the requirement of ubiquitin to detect enzymatic activity in vitro, toxicity for bacterial cells when ubiquitin was provided by a compatible, inducible expression construct, and the ability to cause membrane damage or to be expressed in eukaryotic host cells. Activity enhancement by or binding to liposomes containing PI(4,5)P2 was a variable property suggesting that subfamilies of ExoU-orthologs exist. When enzymes encoded by potential pathogens (Achromobacter, Aeromonas, Legionella, Rickettsia, or Vibrio genera) were biochemically and biologically queried, several were classified as ubiquitin-activated, including enzymes from Achromobacter xylosoxidans and Aeromonas diversa. Based on modeling studies, the ExoU orthologue from A. diversa is structurally and functionally similar to ExoU. In contrast, the enzyme encoded by A. xylosoxidans, AxoU, maintains structural similarity only with the ExoU patatin domain. A. xylosoxidans is an emerging pathogen in cystic fibrosis patients and an opportunistic pathogen in immunocompromised individuals. Pathogenesis of this organism has been associated with a T3SS but the role of AxoU or other effectors is unclear [71].
2.5. ExoU PLA Activity and Pathogenesis
Besides several Pseudomonas species, other bacterial genera utilize PLA activity for pathogenesis. Rickettsia prowazekii protein, RP534, is an ExoU orthologue that possesses PLA1, PLA2, and Lyso-PLA2 activity [40,72,73,74]. R. prowazekii is an obligate intracellular bacterium that causes typhus fever in humans and functions to lyse red blood cells and cell membranes in a PLA-dependent manner [73,74]. Interestingly, the rickettsial ExoU RP534 orthologue only appears to be present in the typhus group of rickettsia. The remainder of the genus encodes another patatin-family PLA enzyme that has also been reported to possess an ExoU-like biochemical regulatory profile [75]. Whether rickettsial patatin-like PLAs are needed for bacterial phagocytosis into the host cell or for phagosome escape and pathogenesis has not been fully elucidated. As a second example, the complete genome sequencing of Bacillus anthracis (Ames strain) revealed three conserved regions that align with the glycine rich motif and serine-aspartate catalytic dyad of ExoU [40,76]. B. anthracis is a spore-forming Gram-positive bacterium and causative agent of anthrax [77]. The major virulence factors of Bacillus anthracis reside on two plasmids, pXO1 and pXO2 [77]. These plasmids encode the secreted exotoxins lethal toxin, protective antigen, and edema factor. Interestingly, the addition of PLA2 inhibitors (e.g., quinacrine) to mouse peritoneal macrophages reduces lethal toxin-mediated cytotoxicity [78]. Well studied PLA2 inhibitors, AACOCF3 (arachidonyl trifluoromethyl ketone, a selective inhibitor of cPLA2) and HELSS ([E]-6-[bromomethylene] tetrahydro-3-[1-naphthalenyl]-2H-pyran-2-one, an irreversible inhibitor of iPLA2), were no more or less potent at reducing cytotoxicity compared to quinacrine [78]. These results suggest that eukaryotic host cPLA2 and iPLA2 are not involved in lethal toxin cytotoxicity. Thus, further studies are needed to fully define the potential regulatory relationship between the B. anthracis ExoU PLA2 orthologue and lethal toxin activity.
2.6. ExoU Subverts the Host Innate Immune Response to Infection
P. aeruginosa ExoU has been associated with severe disease outcomes in animals and humans [10,35]. Infection with an ExoU expressing strain of P. aeruginosa is associated with poor patient outcomes and, in vitro, results in rapid cell death due to lysis. A pathogenic role for ExoU during the initial phase of the pathogen-host interaction has been described. Expression and secretion of ExoU early during the course of infection correlates with increased bacterial burden in the lungs, increased bacterial dissemination, and increased mortality [23,79,80]. Macrophages, neutrophils, epithelial, and endothelial cells are rapidly killed, allowing persistence, proliferation, and dissemination of the bacteria, ultimately contributing to sepsis [13,81]. By intoxicating and killing immune cells, ExoU dysregulates the host innate inflammatory response. Diaz and colleagues demonstrated in a P. aeruginosa-induced pneumonia mouse model that neutrophils are the primary immune cells recruited to the lungs during infection, and these infiltrating neutrophils are intoxicated by ExoU and killed within 90 min [82]. Macrophages are also recruited, injected with ExoU, and killed [83]. In animal infection models, ExoU associates with acute lung epithelial injury, often progressing to bacterial dissemination and septic shock [81,84]. Instillation of an ExoU-encoding strain into rabbit lungs resulted in increased epithelial permeability and leakage of pro-inflammatory mediators into the circulation, along with decreased mean arterial pressure and cardiac output indicative of septic shock. Conversely, animals instilled with a strain lacking ExoU did not exhibit signs of systemic inflammation and septic shock [81]. Furthermore, introducing the gene encoding ExoU into noncytotoxic P. aeruginosa strains expressing a functional T3SS, confers virulence in pneumonia animal models, often resulting in bacterial dissemination [84]. After ExoU has intoxicated and killed innate immune cells and epithelial cells, the last host defense before bacterial dissemination are endothelial cells. Pulmonary endothelial cells are an integral part of the host inflammatory response and function as a barrier between vessels and tissue restricting fluid and protein leak. ExoU has been shown to cause pulmonary endothelial barrier disruption and vasculitis in animal models of pneumonia and Acute Respiratory Distress Syndrome (ARDS) [85,86]. Interactions of T3SS effector proteins with pulmonary endothelial cells post dissemination into the pulmonary circulation are an emerging area of interest.
Recent evidence suggests the P. aeruginosa T3SS and ExoU also subvert a newly emerging facet of the innate immune response to infection. Amyloid-β is best known for its role in the pathology of dementias and Alzheimer’s Disease (AD). Amyloid-β is formed via sequential cleavage of the amyloid precursor protein (APP) by the β-secretase-1 and γ-secretase protease complexes [87]. Depending on the nature of the processing event, differently truncated forms of amyloid-β can be produced (e.g., amyloid-β1–40 or amyloid-β1–42). Subsequent aggregation of amyloid-β leads to the formation of cytotoxic, prion-like fibrils that self-propagate, are long-lived, and form plaques in the brains of Alzheimer’s patients [88,89,90]. Ultimately, cytotoxic amyloid-β aggregates cause neural inflammation and brain endothelial cell (EC) death [91,92,93]. More recently, amyloid-β has been incriminated as a potential contributor to lung pathology associated with pneumonia caused by the ESKAPE pathogens P. aeruginosa, Staphylococcus aureus, and Klebsiella pneumoniae [94]. Indeed, P. aeruginosa-induced pneumonia appears to trigger cytotoxic amyloid-β production in the lung as a pathological mechanism that disrupts pulmonary endothelial cell barrier function [31,33,94,95,96]. However, not all forms of amyloid-β are cytotoxic. Amyloid-β is also an antimicrobial peptide that kills a variety of pathogenic bacteria, viruses, and fungi [97,98,99]. Intriguingly, virulent P. aeruginosa strains expressing the T3SS and exoenzyme effectors such as ExoU inhibit production of antimicrobial amyloid-β and promote formation of cytotoxic amyloid-β as a novel mechanism to subvert the host innate immune response to infection [33]. Current studies are focused on understanding the mechanisms by which ExoU corrupts the production of antimicrobial amyloid-β.
2.7. ExoU Transiently Represses NLRC4 Inflammasome Activation
The innate immune response is a critical first line of defense to invading pathogens or cellular stress. During infection, pathogen- and damage-associated molecular patterns (PAMPs and DAMPs, respectively) are sensed by innate pattern recognition receptors (PRRs), which then assemble with accessory proteins to form an inflammasome. Inflammasomes are multi-protein complexes of PRRs that sense PAMPs and DAMPs produced during infection or other conditions of cellular stress [100]. The nucleotide-binding domain containing leucine rich repeats-like receptor 4 (NLRC4) inflammasome has been shown to recognize P. aeruginosa through interactions between NLR family apoptosis inhibitor proteins (NAIP) receptors and bacterial ligands (e.g., NAIP1-T3SS needle, NAIP2-T3SS rod, and NAIP5/6-flagellin) [101,102,103,104]. In turn, the activated NLRC4 inflammasome complex assembles to subsequently convert pro-caspase-1 into its activated form. Caspase-1 activation results in maturation of IL-1β, IL-18, and the gasderminD executioner of pyroptotic cell death. Activation of pyroptosis and cell death facilitates release of IL-1β, IL-18, and pro-inflammatory DAMPs the extracellular milieu, which then triggers a feed-forward inflammatory response.
A dysregulated host inflammatory response is a hallmark of poor patient outcome progressing to ARDS and sepsis [105,106]. Sutterwala and colleagues first demonstrated recognition of P. aeruginosa by the NLRC4 inflammasome during infection [107]. Importantly, ExoU transiently paralyzes caspase-1 driven NLRC4 inflammasome activation within the first 2 h of P. aeruginosa infection [85,107]. However, the underlying mechanism by which ExoU transiently paralyzes caspase-1 driven NLRC4 inflammasome activation has not been defined. Previous studies have demonstrated a link between P. aeruginosa T3SS, NLRC4 inflammasome, and autophagy. During P. aeruginosa infection, mitochondria become damaged activating the NLRC4 inflammasome. Mitochondria-specific autophagy (mitophagy) is induced in response to mitochondrial damage, which, in turn, down-regulates NLRC4 inflammasome activation [108]. Future studies are required to fully elucidate a potentially novel pathway through which ExoU dysregulates the host inflammatory response during P. aeruginosa infection.
3. Perspectives
P. aeruginosa strains expressing the ExoU PLA2 exoenzyme effector are known to associate with the most adverse patient outcomes, likely owing to the ability of ExoU to subvert host innate immune responses and cause cellular damage. Virulence factors, such as the T3SS and ExoU, represent the next frontier of precision medicine therapeutic targets to combat the imminent threat of antibiotic resistant infectious agents such the ESKAPE pathogens. ExoU is an especially attractive potential target, considering its contributions to P. aeruginosa virulence and its requirement for a eukaryotic-specific protein, ubiquitin, to fully hydrolyze membrane substrates. As the substrate specificity of ExoU is relatively broad, the interaction between ExoU and SpcU, as well as the requirement for interaction with ubiquitin for enzyme activation, are postulated to protect P. aeruginosa from the membrane destructive effects of ExoU prior to its direct injection into host cells by the T3SS. Thus, interventions that activate ExoU in the bacteria or inactivate ExoU in the host could mitigate P. aeruginosa infection. In addition, ExoU orthologs and related enzymes that also require similar activation steps are encoded by a variety of Gram-negative bacteria that are pathogenic for humans. Understanding the structural transitions between inactive and active states of these proteins may provide a target for rationally designed ligands that can prevent this conversion to a cell and tissue destructive virulence factor. The availability of crystal structures and recent use of biophysical and computational modeling techniques [54] open new horizons for understanding allosteric changes of dynamic enzymes in response to both cofactors and substrates.
Acknowledgments
The Audia laboratory would like to thank Nicole A. Housley for excellent technical support. The Frank laboratory would like to thank Molly O. Riegert for excellent technical support.
Author Contributions
Writing—original draft preparation, K.S.H.; writing—review and editing, K.S.H., M.H.T., D.W.F., J.P.A.; funding acquisition, K.S.H., D.W.F., J.P.A. All authors have read and agreed to the published version of the manuscript.
Funding
The work presented herein was funded by NIH/NHLBI grant R01 HL118334 (JPA) and R01 HL118334-S1 (KSH), and NIH/NIAID grant R01 AI104922 (DWF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This article is a high-level review of the relationships between structure, function, and virulence capacity of the P. aeruginosa type III secretion system effector, ExoU. It is well-established that ExoU is a cytotoxic phospholipase A2, which subverts the host innate immune response to infection and associates with poor patient outcomes. Therefore, ExoU is a potential target for the development of novel antimicrobial therapeutics.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feldman M., Bryan R., Rajan S., Scheffler L., Brunnert S., Tang H., Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 1998;66:43–51. doi: 10.1128/IAI.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lister P.D., Wolter D.J., Hanson N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. [(accessed on 27 February 2017)]. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1.
- 4.Pendleton J.N., Gorman S.P., Gilmore B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 5.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 6.Brewer S.C., Wunderink R.G., Jones C.B., Leeper K.V., Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest. 1996;109:1019–1029. doi: 10.1378/chest.109.4.1019. [DOI] [PubMed] [Google Scholar]
- 7.ElSolh A.A., Akinnusi M.E., Wiener-Kronish J.P., Lynch S.V., Pineda L.A., Szarpa K. Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2008;178:513–519. doi: 10.1164/rccm.200802-239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Solh A.A., Hattemer A., Hauser A.R., Alhajhusain A., Vora H. Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit. Care Med. 2012;40:1157–1163. doi: 10.1097/CCM.0b013e3182377906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser A.R., Cobb E., Bodi M., Mariscal D., Valles J., Engel J.N., Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Juan C., Pena C., Oliver A. Host and pathogen biomarkers for severe Pseudomonas aeruginosa infections. J. Infect. Dis. 2017;215((Suppl. S1)):S44–S51. doi: 10.1093/infdis/jiw299. [DOI] [PubMed] [Google Scholar]
- 11.Sadikot R.T., Blackwell T.S., Christman J.W., Prince A.S. Pathogen–Host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B.J., Dehnbostel J., Blackwell T.S. Pseudomonas aeruginosa: Host defence in lung diseases. Respirology. 2010;15:1037–1056. doi: 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- 13.Gellatly S.L., Hancock R.E. Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog. Dis. 2013;67:159–173. doi: 10.1111/2049-632X.12033. [DOI] [PubMed] [Google Scholar]
- 14.Roy-Burman A., Savel R.H., Racine S., Swanson B.L., Revadigar N.S., Fujimoto J., Sawa T., Frank D.W., Wiener-Kronish J.P. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- 15.Tsay T.B., Jiang Y.Z., Hsu C.M., Chen L.W. Pseudomonas aeruginosa colonization enhances ventilator-associated pneumonia-induced lung injury. Respir. Res. 2016;17:101. doi: 10.1186/s12931-016-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudoh I., Wiener-Kronish J.P., Hashimoto S., Pittet J.F., Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am. J. Physiol. 1994;267:L551–L556. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- 17.Wiener-Kronish J.P., Sakuma T., Kudoh I., Pittet J.F., Frank D., Dobbs L., Vasil M.L., Matthay M.A. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J. Appl. Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661. [DOI] [PubMed] [Google Scholar]
- 18.Ader F., LeBerre R., Faure K., Gosset P., Epaulard O., Toussaint B., Polack B., Nowak E., Viget N.B., Kipnis E., et al. Alveolar response to Pseudomonas aeruginosa: Role of the type III secretion system. Infect. Immun. 2005;73:4263–4271. doi: 10.1128/IAI.73.7.4263-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dacheux D., Attree I., Schneider C., Toussaint B. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect. Immun. 1999;67:6164–6167. doi: 10.1128/IAI.67.11.6164-6167.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morin C.D., Deziel E., Gauthier J., Levesque R.C., Lau G.W. An organ system-based synopsis of Pseudomonas aeruginosa virulence. Virulence. 2021;12:1469–1507. doi: 10.1080/21505594.2021.1926408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds D., Kollef M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs. 2021;81:2117–2131. doi: 10.1007/s40265-021-01635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawa T. The molecular mechanism of acute lung injury caused by Pseudomonas aeruginosa: From bacterial pathogenesis to host response. J. Intensive Care. 2014;2:10. doi: 10.1186/2052-0492-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulert G.S., Feltman H., Rabin S.D.P., Martin C.G., Battle S.E., Rello J., Hauser A.R. Secretion of the Toxin ExoU Is a Marker for Highly Virulent Pseudomonas aeruginosa Isolates Obtained from Patients with Hospital-Acquired Pneumonia. J. Infect. Dis. 2003;188:1695–1706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- 24.Fleiszig S.M., Wiener-Kronish J.P., Miyazaki H., Vallas V., Mostov K.E., Kanada D., Sawa T., Yen T.S., Frank D.W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feltman H., Schulert G., Khan S., Jain M., Peterson L., Hauser A.R. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 26.Barbieri J.T., Sun J. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 2004;152:79–92. doi: 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 27.Hauser A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Genet. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckert U., Wolter S., Hartwig C., Bähre H., Kaever V., Ladant D., Frank D.W., Seifert R. ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochem. Biophys. Res. Commun. 2014;450:870–874. doi: 10.1016/j.bbrc.2014.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belyy A., Raoux-Barbot D., Saveanu C., Namane A., Ogryzko V., Worpenberg L., David V., Henriot V., Fellous S., Merrifield C., et al. Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins. Nat. Commun. 2016;7:13582. doi: 10.1038/ncomms13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow K.A., Frank D.W., Balczon R., Stevens T. The Pseudomonas aeruginosa Exoenzyme Y: A Promiscuous Nucleotidyl Cyclase Edema Factor and Virulence Determinant. Non-Canonical Cycl. Nucleotides. 2016;238:67–85. doi: 10.1007/164_2016_5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrow K.A., Ochoa C.D., Balczon R., Zhou C., Cauthen L., Alexeyev M., Schmalzer K.M., Frank D.W., Stevens T. Pseudomonas aeruginosa exoenzymes U and Y induce a transmissible endothelial proteinopathy. Am. J. Physiol. Cell. Mol. Physiol. 2016;310:L337–L353. doi: 10.1152/ajplung.00103.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayner S.L., Frank D.W., King J., Chen H., VandeWaa J., Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ. Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 33.Voth S., Gwin M., Francis C.M., Balczon R., Frank D.W., Pittet J., Wagener B.M., Moser S.A., Alexeyev M., Housley N., et al. Virulent Pseudomonas aeruginosa infection converts antimicrobial amyloids into cytotoxic prions. FASEB J. 2020;34:9156–9179. doi: 10.1096/fj.202000051RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finck-Barbançon V., Goranson J., Zhu L., Sawa T., Wiener-Kronish J.P., Fleiszig S.M.J., Wu C., Mende-Mueller L., Frank D.W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 35.Fischer S., Dethlefsen S., Klockgether J., Tummler B. Phenotypic and Genomic Comparison of the Two Most Common ExoU-Positive Pseudomonas aeruginosa Clones, PA14 and ST235. Msystems. 2020;5:e01007-20. doi: 10.1128/mSystems.01007-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauser A.R., Kang P.J., Engel J.N. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 37.Kulasekara B.R., Kulasekara H.D., Wolfgang M.C., Stevens L., Frank D.W., Lory S. Acquisition and Evolution of the exoU Locus in Pseudomonas aeruginosa. J. Bacteriol. 2006;188:4037–4050. doi: 10.1128/JB.02000-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato H., Frank D.W., Hillard C.J., Feix J.B., Pankhaniya R.R., Moriyama K., Finck-Barbançon V., Buchaklian A., Lei M., Long R.M., et al. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips R.M., Six D., Dennis E.A., Ghosh P., Matsumoto K., Shionyu M., Go M., Shimizu K., Shinomura T., Kimata K., et al. In Vivo Phospholipase Activity of the Pseudomonas aeruginosa Cytotoxin ExoU and Protection of Mammalian Cells with Phospholipase A2 Inhibitors. J. Biol. Chem. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- 40.Sato H., Frank D.W. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 41.Finck-Barbançon V., Frank D.W. Multiple Domains Are Required for the Toxic Activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 2001;183:4330–4344. doi: 10.1128/JB.183.14.4330-4344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirschberg H.J.H.B., Simons J.-W.F.A., Dekker N., Egmond M.R. Cloning, expression, purification and characterization of patatin, a novel phospholipase A. JBIC J. Biol. Inorg. Chem. 2001;268:5037–5044. doi: 10.1046/j.0014-2956.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 43.Dessen A., Tang J., Schmidt H., Stahl M., Clark J.D., Seehra J., Somers W.S. Crystal Structure of Human Cytosolic Phospholipase A2 Reveals a Novel Topology and Catalytic Mechanism. Cell. 1999;97:349–360. doi: 10.1016/S0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- 44.Rabin S.D.P., Hauser A.R. Functional Regions of the Pseudomonas aeruginosa Cytotoxin ExoU. Infect. Immun. 2005;73:573–582. doi: 10.1128/IAI.73.1.573-582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finck-Barbançon V., Yahr T.L., Frank D.W. Identification and Characterization of SpcU, a Chaperone Required for Efficient Secretion of the ExoU Cytotoxin. J. Bacteriol. 1998;180:6224–6231. doi: 10.1128/JB.180.23.6224-6231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabin S.D.P., Veesenmeyer J.L., Bieging K.T., Hauser A.R. A C-Terminal Domain Targets the Pseudomonas aeruginosa Cytotoxin ExoU to the Plasma Membrane of Host Cells. Infect. Immun. 2006;74:2552–2561. doi: 10.1128/IAI.74.5.2552-2561.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmalzer K.M., Benson M.A., Frank D.W. Activation of ExoU Phospholipase Activity Requires Specific C-Terminal Regions. J. Bacteriol. 2010;192:1801–1812. doi: 10.1128/JB.00904-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veesenmeyer J.L., Howell H., Halavaty A.S., Ahrens S., Anderson W.F., Hauser A.R. Role of the Membrane Localization Domain of the Pseudomonas aeruginosa Effector Protein ExoU in Cytotoxicity. Infect. Immun. 2010;78:3346–3357. doi: 10.1128/IAI.00223-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stirling F.R., Cuzick A., Kelly S.M., Oxley D., Evans T.J. Eukaryotic localization, activation and ubiquitinylation of a bacterial type III secreted toxin. Cell. Microbiol. 2006;8:1294–1309. doi: 10.1111/j.1462-5822.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 50.Benson M.A., Komas S.M., Schmalzer K.M., Casey M.S., Frank D.W., Feix J.B. Induced Conformational Changes in the Activation of the Pseudomonas aeruginosa type III Toxin, ExoU. Biophys. J. 2011;100:1335–1343. doi: 10.1016/j.bpj.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gendrin C., Contreras-Martel C., Bouillot S., Elsen S., Lemaire D., Skoufias D., Huber P., Attree I., Dessen A. Structural Basis of Cytotoxicity Mediated by the Type III Secretion Toxin ExoU from Pseudomonas aeruginosa. PLoS Pathog. 2012;8:e1002637. doi: 10.1371/journal.ppat.1002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halavaty A.S., Borek D., Tyson G., Veesenmeyer J.L., Shuvalova L., Minasov G., Otwinowski Z., Hauser A.R., Anderson W.F. Structure of the Type III Secretion Effector Protein ExoU in Complex with Its Chaperone SpcU. PLoS ONE. 2012;7:e49388. doi: 10.1371/journal.pone.0049388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akeda Y., Galán J.E. Chaperone release and unfolding of substrates in type III secretion. Nat. Cell Biol. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- 54.Tessmer M.H., DeCero S.A., Del Alamo D., Riegert M.O., Meiler J., Frank D.W., Feix J.B. Characterization of the ExoU activation mechanism using EPR and integrative modeling. Sci. Rep. 2020;10:19700. doi: 10.1038/s41598-020-76023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato H., Feix J.B., Frank D.W. Identification of Superoxide Dismutase as a Cofactor for thePseudomonasType III Toxin, ExoU. Biochem. 2006;45:10368–10375. doi: 10.1021/bi060788j. [DOI] [PubMed] [Google Scholar]
- 56.Benson M.A., Schmalzer K.M., Frank D.W. A sensitive fluorescence-based assay for the detection of ExoU-mediated PLA2 activity. Clin. Chim. Acta. 2010;411:190–197. doi: 10.1016/j.cca.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson D.M., Schmalzer K.M., Sato H., Casey M., Terhune S.S., Haas A.L., Feix J.B., Frank D.W. Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU. Mol. Microbiol. 2011;82:1454–1467. doi: 10.1111/j.1365-2958.2011.07904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annul. Rev. Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- 59.Anderson D.M., Feix J.B., Monroe A.L., Peterson F.C., Volkman B.F., Haas A.L., Frank D.W. Identification of the Major Ubiquitin-binding Domain of the Pseudomonas aeruginosa ExoU A2 Phospholipase. J. Biol. Chem. 2013;288:26741–26752. doi: 10.1074/jbc.M113.478529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tessmer M.H., Anderson D.M., Buchaklian A., Frank D.W., Feix J.B. Cooperative Substrate-Cofactor Interactions and Membrane Localization of the Bacterial Phospholipase A2 (PLA2) Enzyme, ExoU. J. Biol. Chem. 2017;292:3411–3419. doi: 10.1074/jbc.M116.760074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tessmer M.H., Anderson D.M., Pickrum A.M., Riegert M.O., Moretti R., Meiler J., Feix J.B., Frank D.W. Identification of a ubiquitin-binding interface using Rosetta and DEER. Proc. Natl. Acad. Sci. USA. 2018;115:525–530. doi: 10.1073/pnas.1716861115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyson G., Halavaty A.S., Kim H., Geissler B., Agard M., Satchell K.J., Cho W., Anderson W.F., Hauser A.R. A Novel Phosphatidylinositol 4,5-Bisphosphate Binding Domain Mediates Plasma Membrane Localization of ExoU and Other Patatin-like Phospholipases. J. Biol. Chem. 2015;290:2919–2937. doi: 10.1074/jbc.M114.611251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyson G.H., Hauser A.R. Phosphatidylinositol 4,5-Bisphosphate Is a Novel Coactivator of the Pseudomonas aeruginosa Cytotoxin ExoU. Infect. Immun. 2013;81:2873–2881. doi: 10.1128/IAI.00414-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang A., Veesenmeyer J.L., Hauser A.R. Phosphatidylinositol 4,5-Bisphosphate-Dependent Oligomerization of the Pseudomonas aeruginosa Cytotoxin ExoU. Infect. Immun. 2018;86:e00402-17. doi: 10.1128/IAI.00402-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLaughlin S., Wang J., Gambhir A., Murray D. PIP2 and Proteins: Interactions, Organization, and Information Flow. Annu. Rev. Biophys. Biomol. Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 66.Anderson D.M., Sato H., Dirck A.T., Feix J.B., Frank D.W. Ubiquitin Activates Patatin-Like Phospholipases from Multiple Bacterial Species. J. Bacteriol. 2015;197:529–541. doi: 10.1128/JB.02402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato H., Frank D.W. Intoxication of Host Cells by the T3SS Phospholipase ExoU: PI(4,5)P2-Associated, Cytoskeletal Collapse and Late Phase Membrane Blebbing. PLoS ONE. 2014;9:e103127. doi: 10.1371/journal.pone.0103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee V.T., Pukatzki S., Sato H., Kikawada E., Kazimirova A.A., Huang J., Li X., Arm J.P., Frank D.W., Lory S. Pseudolipasin A Is a Specific Inhibitor for Phospholipase A 2 Activity of Pseudomonas aeruginosa Cytotoxin ExoU. Infect. Immun. 2007;75:1089–1098. doi: 10.1128/IAI.01184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim D., Baek J., Song J., Byeon H., Min H., Min K.H. Identification of arylsulfonamides as ExoU inhibitors. Bioorganic Med. Chem. Lett. 2014;24:3823–3825. doi: 10.1016/j.bmcl.2014.06.064. [DOI] [PubMed] [Google Scholar]
- 70.Foulkes D.M., McLean K., Haneef A., Fernig D.G., Winstanley C., Berry N., Kaye S.B. Pseudomonas aeruginosa Toxin ExoU as a Therapeutic Target in the Treatment of Bacterial Infections. Microorganisms. 2019;7:707. doi: 10.3390/microorganisms7120707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pickrum A.M., DeLeon O., Dirck A., Tessmer M.H., Riegert M.O., Biller J.A., Ledeboer N.A., Kirby J.R., Frank D.W. Achromobacter xylosoxidans Cellular Pathology Is Correlated with Activation of a Type III Secretion System. Infect. Immun. 2020;88 doi: 10.1128/IAI.00136-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Housley N.A., Winkler H.H., Audia J.P. The Rickettsia prowazekii ExoU Homologue Possesses Phospholipase A 1 (PLA 1), PLA 2, and Lyso-PLA 2 Activities and Can Function in the Absence of Any Eukaryotic Cofactors In Vitro. J. Bacteriol. 2011;193:4634–4642. doi: 10.1128/JB.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winkler H.H., Miller E.T. Phospholipase A Activity in the Hemolysis of Sheep and Human Erythrocytes by Rickettsia prowazeki. Infect. Immun. 1980;29:316–321. doi: 10.1128/iai.29.2.316-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winkler H.H., Miller E.T. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells) Infect. Immun. 1982;38:109–113. doi: 10.1128/iai.38.1.109-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blanc G., Renesto P., Raoult D. Phylogenic Analysis of Rickettsial Patatin-like Protein with Conserved Phospholipase A2 Active Sites. Ann. N. Y. Acad. Sci. 2005;1063:83–86. doi: 10.1196/annals.1355.012. [DOI] [PubMed] [Google Scholar]
- 76.Read T., Peterson S.N., Tourasse N., Baillie L., Paulsen I., Nelson K.E., Tettelin H., Fouts D.E., Eisen J.A., Gill S.R., et al. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nat. Cell Biol. 2003;423:81–86. doi: 10.1038/nature01586. [DOI] [PubMed] [Google Scholar]
- 77.Dixon T.C., Meselson M., Guillemin J., Hanna P.C. Anthrax. N. Engl. J. Med. 1999;341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 78.Shin S., Kim Y.-B., Hur G.-H. Involvement of phospholipase A2 activation in anthrax lethal toxin-induced cytotoxicity. Cell Biol. Toxicol. 1999;15:19–29. doi: 10.1023/A:1007546505528. [DOI] [PubMed] [Google Scholar]
- 79.Howell H.A., Logan L.K., Hauser A.R. Type III Secretion of ExoU Is Critical during Early Pseudomonas aeruginosa Pneumonia. MBio. 2013;4:e00032-13. doi: 10.1128/mBio.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaver C.M., Hauser A.R. Relative Contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to Virulence in the Lung. Infect. Immun. 2004;72:6969–6977. doi: 10.1128/IAI.72.12.6969-6977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kurahashi K., Kajikawa O., Sawa T., Ohara M., Gropper M.A., Frank D.W., Martin T.R., Wiener-Kronish J.P. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 1999;104:743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diaz M.H., Shaver C.M., King J.D., Musunuri S., Kazzaz J.A., Hauser A.R. Pseudomonas aeruginosa Induces Localized Immunosuppression during Pneumonia. Infect. Immun. 2008;76:4414–4421. doi: 10.1128/IAI.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diaz M.H., Hauser A.R. Pseudomonas aeruginosa Cytotoxin ExoU Is Injected into Phagocytic Cells during Acute Pneumonia. Infect. Immun. 2010;78:1447–1456. doi: 10.1128/IAI.01134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allewelt M., Coleman F.T., Grout M., Priebe G.P., Pier G.B. Acquisition of Expression of the Pseudomonas aeruginosa ExoU Cytotoxin Leads to Increased Bacterial Virulence in a Murine Model of Acute Pneumonia and Systemic Spread. Infect. Immun. 2000;68:3998–4004. doi: 10.1128/IAI.68.7.3998-4004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alvarez D.F., Housley N., Koloteva A., Zhou C., O’Donnell K., Audia J.P. Caspase-1 Activation Protects Lung Endothelial Barrier Function during Infection-Induced Stress. Am. J. Respir. Cell Mol. Biol. 2016;55:500–510. doi: 10.1165/rcmb.2015-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindsey A.S., Sullivan L.M., Housley N.A., Koloteva A., King J.A., Audia J.P., Alvarez D.F. Analysis of pulmonary vascular injury and repair during Pseudomonas aeruginosa infection-induced pneumonia and acute respiratory distress syndrome. Pulm. Circ. 2019;9:2045894019826941. doi: 10.1177/2045894019826941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thinakaran G., Koo E.H. Amyloid Precursor Protein Trafficking, Processing, and Function. J. Biol. Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sevigny J., Chiao P., Bussiere T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 89.Inestrosa N.C., Soto C. Molecular biology of the amyloid of Alzheimer’s disease. An overview. Biol. Res. 1992;25:63–72. [PubMed] [Google Scholar]
- 90.Du D., Murray A.N., Cohen E., Kim H.E., Simkovsky R., Dillin A., Kelly J.W. A kinetic aggregation assay allowing selective and sensitive amyloid-beta quantification in cells and tissues. Biochemistry. 2011;50:1607–1617. doi: 10.1021/bi1013744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parodi-Rullán R., Sone J.Y., Fossati S. Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. J. Alzheimer’s Dis. 2019;72:1019–1039. doi: 10.3233/JAD-190357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dempsey C., Rubio Araiz A., Bryson K.J., Finucane O., Larkin C., Mills E.L., Robertson A.A.B., Cooper M.A., O’Neill L.A.J., Lynch M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav. Immun. 2017;61:306–316. doi: 10.1016/j.bbi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 93.Price J.M., Chi X., Hellermann G., Sutton E.T. Physiological levels of beta-amyloid induce cerebral vessel dysfunction and reduce endothelial nitric oxide production. Neurol. Res. 2001;23:506–512. doi: 10.1179/016164101101198758. [DOI] [PubMed] [Google Scholar]
- 94.Lin M.T., Balczon R., Pittet J.-F., Wagener B.M., Moser S.A., Morrow K.A., Voth S., Francis C.M., Leavesley S., Bell J., et al. Nosocomial Pneumonia Elicits an Endothelial Proteinopathy: Evidence for a Source of Neurotoxic Amyloids in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2018;198:1575–1578. doi: 10.1164/rccm.201801-0060LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balczon R., Morrow K.A., Zhou C., Edmonds B., Alexeyev M., Pittet J., Wagener B.M., Moser S.A., Leavesley S., Zha X., et al. Pseudomonas aeruginosa infection liberates transmissible, cytotoxic prion amyloids. FASEB J. 2017;31:2785–2796. doi: 10.1096/fj.201601042RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stevens T.C., Ochoa C.D., Morrow K.A., Robson M.J., Prasain N., Zhou C., Alvarez D.F., Frank D.W., Balczon R., Stevens T. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2014;306:L915–L924. doi: 10.1152/ajplung.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumar D.K.V., Choi S.H., Washicosky K.J., Eimer W.A., Tucker S., Ghofrani J., Lefkowitz A., McColl G., Goldstein L.E., Tanzi R.E., et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci. Transl. Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar D.K., Eimer W.A., Tanzi R.E., Moir R.D. Alzheimer’s disease: The potential therapeutic role of the natural antibiotic amyloid-β peptide. Neurodegener. Dis. Manag. 2016;6:345–348. doi: 10.2217/nmt-2016-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soscia S.J., Kirby J.E., Washicosky K.J., Tucker S.M., Ingelsson M., Hyman B., Burton M.A., Goldstein L.E., Duong S., Tanzi R.E., et al. The Alzheimer’s disease-associated amyloid β-protein is an antimicrobial peptide. PLoS ONE. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 101.Grandjean T., Boucher A., Thepaut M., Monlezun L., Guery B., Faudry E., Kipnis E., Dessein R. The human NAIP-NLRC4-inflammasome senses the Pseudomonas aeruginosa T3SS inner-rod protein. Int. Immunol. 2017;29:377–384. doi: 10.1093/intimm/dxx047. [DOI] [PubMed] [Google Scholar]
- 102.Kofoed E.M., Vance R.E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nat. Cell Biol. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rayamajhi M., Zak D.E., Chavarria-Smith J., Vance R.E., Miao E.A. Cutting Edge: Mouse NAIP1 Detects the Type III Secretion System Needle Protein. J. Immunol. 2013;191:3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Y., Yang J., Shi J., Gong Y.-N., Lu Q., Xu H., Liu L., Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nat. Cell Biol. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 105.Dolinay T., Kim Y.S., Howrylak J., Hunninghake G.M., An C.H., Fredenburgh L., Massaro A.F., Rogers A., Gazourian L., Nakahira K., et al. Inflammasome-regulated Cytokines Are Critical Mediators of Acute Lung Injury. Am. J. Respir. Crit. Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sutterwala F.S., Mijares L.A., Li L., Ogura Y., Kazmierczak B.I., Flavell R.A. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ullah I., Bayes H.K., Li D., Simon A.K., Jabir M.S., Hopkins L., Ritchie N.D., Tourlomousis P., Lupton A., Puleston D., et al. Mitochondrial damage contributes to Pseudomonas aeruginosa activation of the inflammasome and is downregulated by autophagy. Autophagy. 2015;11:166–182. doi: 10.6084/m9.figshare.1314127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.