Abstract
Patients with end-stage kidney disease represent a frail population and might be at higher risk of SARS-CoV-2 infection. The Lazio Regional Dialysis and Transplant Registry collected information on dialysis patients with a positive swab. The study investigated incidence of SARS-CoV-2 infection, mortality and their potential associated factors in patients undergoing maintenance hemodialysis (MHD) in the Lazio region. Method: The occurrence of infection was assessed among MHD patients included in the RRDTL from 1 March to 30 November 2020. The adjusted cumulative incidence of infection and mortality risk within 30 days of infection onset were estimated. Logistic and Cox regression models were applied to identify factors associated with infection and mortality, respectively. Results: The MHD cohort counted 4942 patients; 256 (5.2%) had COVID-19. The adjusted cumulative incidence was 5.1%. Factors associated with infection included: being born abroad, educational level, cystic renal disease/familial nephropathy, vascular disease and being treated in a dialysis center located in Local Health Authority (LHA) Rome 2. Among infected patients, 59 (23.0%) died within 30 days; the adjusted mortality risk was 21.0%. Factors associated with 30-day mortality included: age, malnutrition and fever at the time of swab. Conclusions: Factors associated with infection seem to reflect socioeconomic conditions. Factors associated with mortality, in addition to age, are related to clinical characteristics and symptoms at the time of swab.
Keywords: hemodialysis, end-stage kidney disease, SARS-CoV-2 infection, COVID-19, incidence, mortality, outcome
1. Introduction
A novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), appeared in late 2019 in Wuhan, China, and rapidly spread progressively to other countries [1,2,3], with the WHO declaring it a pandemic and public health emergency of international concern in March 2020 [4]. As of 31 December 2020, 190 countries had confirmed cases, with Italy ranking eighth with 2,107,166 cumulative cases and 74,159 deaths [5]. According to the latest published data, in Rome, 5750 deaths in individuals positive to the SARS-CoV-2 occurred between 27 January 2020 and 6 April 2021 [6].
COVID-19 primarily manifests itself as an acute upper and lower respiratory tract illness that may be complicated by interstitial and alveolar pneumonia. It may also affect multiple other tissues such as the heart, digestive tract, kidneys, blood and nervous system [7,8,9]. According to the published literature, older patients with underlying chronic conditions such as diabetes mellitus, hypertension and cardiovascular disease tend to be more susceptible to COVID-19 and become severely ill [10]. To date, COVID-19 has caused a significant increase in the number of hospitalizations and intensive care unit admissions, with now well-investigated pulmonary, cardiac, vascular and renal complications [11,12,13,14,15].
The emergence of this pandemic put an additional strain on vulnerable population subgroups, such as end-stage chronic kidney disease (ESKD) patients, especially those on maintenance hemodialysis (MHD) [16].
MHD patients are at increased risk for COVID-19 infection and its complications for several reasons, primarily due to their dysregulated immune system because of their uremic state [17]. This is often accompanied by significant comorbidities such as cardiovascular disease, obstructive pulmonary disease, diabetes mellitus, obesity and cerebrovascular disease [18,19]. The logistical aspects of MHD treatment further increase the risk of SARS-CoV-2 infection; indeed, they require caregiver assistance and transportation from home to the dialysis unit and stay with dozens of patients undergoing MHD in a dialysis unit for several hours at a time, which may lead to widespread cross-contamination [20]. Hence, the management of MHD patients in the context of the pandemic presents several challenges and, in response, several guidelines aimed at mitigating the risk of infection spread in outpatient hemodialysis facilities have been already published [21,22,23,24,25].
Despite recent improvements in life expectancy among patients with ESKD, with a 28% decline in mortality over the last 16 years, the ESKD population has a higher mortality rate compared with the general population even after adjusting for age, race and diabetes mellitus [26]. With COVID-19, studies, including case reports or the experience of dialysis centers, suggest a more severe course of the disease and an increased risk of death in patients with chronic kidney disease, especially among those undergoing MHD [16,27,28,29,30,31,32,33,34,35,36,37].
To date, few studies have been conducted using ad hoc data on SARS-CoV-2 infection among MHD patients retrieved from dialysis registries [38,39].
The Lazio Regional Dialysis and Transplant Registry (RRDTL) was extended to include a new section on SARS-CoV-2 infection among patients undergoing dialysis treatment in the Lazio region. Information was retrieved through a questionnaire that collected information and monitored the health status of patients related to COVID-19 [40,41].
The aim of our study was to investigate the health effects of SARS-CoV-2 infection among MHD patients in the Lazio region, specifically considering incidence of SARS-CoV-2 infection, mortality within 30 days of infection onset and factors associated with the two outcomes in MHD patients.
2. Materials and Methods
2.1. Data Sources
The Lazio Regional Dialysis and Transplant Registry (RRDTL) is a population-based registry established in 1994 that collects detailed information on all patients undergoing chronic dialysis (e.g., those undergoing either hemodialysis or peritoneal dialysis for a period of at least 90 days). Information on sociodemographic status, clinical characteristics, dialysis treatments and drug therapy is collected for patients treated in all public and private accredited dialysis centers of the Lazio region in Italy. All dialysis units of the Lazio region are requested, by law, to register the information on their patients and to update the information every 6 months. If the patient no longer receives dialysis at the centers, the dialysis unit is required to communicate the date of termination of the dialysis treatment and the reason for the termination (kidney transplant, renal recovery, transfer to another dialysis center, death). [42] Details on the RRDTL are reported elsewhere [43,44,45,46,47]. In 2019, the RRDTL collected information on around 5000 dialysis patients [42].
A specific section, named “RRDTL COVID-19 section”, collecting information on SARS-CoV-2 infection, was included in the RRDTL, starting from March 2020. Data were collected for all dialysis patients who had a positive nasopharyngeal swab specimen to detect SARS-CoV-2 either in case of onset of symptoms or contact with a suspected or infected case of SARS-CoV-2 according to regional/national guidelines [21,22,23,24,25]. Information on symptoms on the day of infection (date of the first positive swab), need for hospitalization, complications and the end of infection (the date of the negative swab or the date of death), was collected.
The Lazio regional mortality registry was used to confirm the date of death registered in the RRDTL [48].
Moreover, to assess the data accuracy of the “RRDTL COVID-19 section”, MHD patients included in the study were linked through a record linkage procedure with an anonymous coding system with the regional “Emergenza CoronaVirus” platform for the study period (1 March–30 November 2020). In cases where a patient was found to have a positive swab only in the regional platform, the nephrologist in charge of the patient was contacted to clarify the patient’s status in terms of SARS-CoV-2 infection. If the infection status was confirmed, the nephrologist was asked to fill in the “RRDTL COVID-19 section”. The study population of infected patients was comprised of those that had the “RRDTL COVID-19 section” compiled [49].
2.2. Study Design and Population
A population-based prospective cohort study was performed. The cohort included all MHD patients resident in the Lazio region (over 5,700,000 residents), aged 18 years and over, treated between 1 March and 31 October 2020.
Cases between 1 March and 30 November 2020 were included in the study to assess infection status, and each infected patient was followed for 30 days from the infection onset date (date of first positive swab) to investigate 30-day mortality.
The outcomes investigated were: (1) SARS-CoV-2 infection between 1 March and 30 November 2020; (2) 30-day mortality among the infected patients.
2.3. Covariates
The following sociodemographic, clinical and treatment variables were recorded and tested as potential factors associated with the incidence of SARS-CoV-2 infection and 30-day mortality after infection: gender, age (18–64, 65–84, ≥85 years old), place of birth (abroad, Italy), educational level (illiterate, from 1–8 years of study, ≥9 years), self-sufficiency (total autonomy, autonomy in some activities, non-self-sufficient), type of nephropathy (glomerulonephritis, interstitial and toxic nephritis/pyelonephritis, cystic renal disease or familial nephropathy, renal malformation, renal vascular disease, diabetic nephropathy, systemic disease, unknown, other nephropathies; yes, no), comorbidities (severe hypertension, heart disease, diabetes, vascular disease, cerebrovascular disease, chronic pulmonary disease (COPD), cancer, thyroid disease, lipid metabolism’s alteration, motor deficit, liver disease, extra-uremic anemia, chronic inflammatory bowel disease (IBD), peptic ulcer, dementia, psychiatric disease, malnutrition, obesity, renal transplant; yes, no), local health authority (LHA) of dialysis center (named: Roma 1, Roma 2, Roma 3, Roma 4, Roma 5, Roma 6, Viterbo, Rieti, Latina and Frosinone), hours per week of dialysis (from 3–9, ≥10), hemodialysis vintage (one year or less, ≥2 years).
For patients with SARS-CoV-2 who died within 30 days from the infection onset, the following variables were also tested: infection time onset (March-July, August-November), symptoms at the time of swab (fever, cough, colds, conjunctivitis, respiratory distress, loss of taste and smell, gastrointestinal disease; yes, no), hospitalization (yes, no).
2.4. Statistical Analysis
To investigate the incidence of the SASR-CoV-2, infection variables were displayed according to infection status and χ2 test was used to test statistical significance of differences between subgroups (p-value < 0.05).
Univariate and multivariable log-binomial regression models were performed to estimate the crude and the age- and gender-adjusted risk of SARS-CoV-2 infection along with their 95% CI.
A multivariable logistic regression model, with a stepwise selection for the variables associated at univariate level with SARS-CoV-2 infection, was fitted to identify the potential factors associated with infection status (odds ratio—OR; 95% confidence interval—95% CI).
To investigate the 30-day mortality of infection onset, all variables were displayed according to vital status within 30 days of infection onset and χ2 test was used to test statistical significance of differences between subgroups (p-value < 0.05).
Univariate and multivariable log-binomial regression models were performed to estimate the crude and the age- and gender-adjusted risk of 30-day mortality along with their 95% CI.
A Cox regression model with a stepwise selection for the variables associated at univariate level with 30-day mortality was fitted to identify potential factors associated with 30-day mortality (hazard ratio—HR; 95% CI).
All analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC, USA). This study was carried out in full compliance with the current privacy laws.
3. Results
3.1. Characteristics of the Study Population by Infection Status
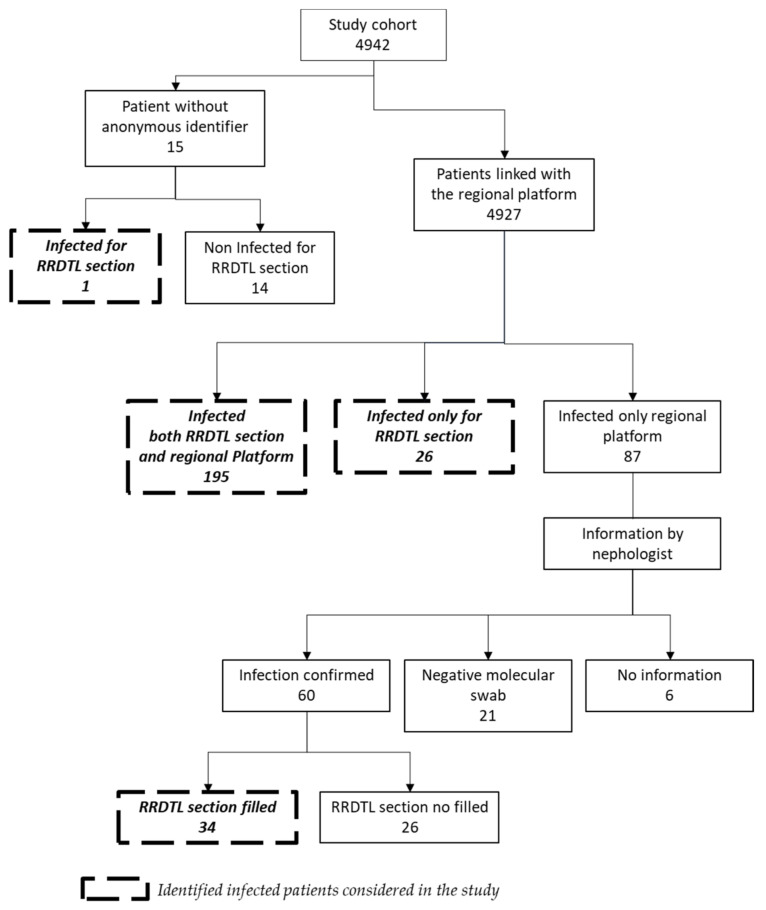
The cohort included 4942 MHD patients: 65.5% male, with an average age of 68.5 years (STD: 14.2 years). Of these, 4927 had the regional anonymous identifier necessary to carry out the record linkage procedures with the regional platform to verify SARS-CoV-2 infection. The “RRDTL COVID-19 section” identified 222 patients with a positive SARS-CoV-2 swab; among them, one could not be searched in the platform since he did not have an anonymous code, 195 (88%) were also found in the regional platform and 26 (12%) were not. The regional platform identified a further 87 MHD patients with a positive nasopharyngeal swab, and the patients’ nephrologists confirmed SARS-CoV-2 infection for 60 (69%) of them. The “RRDTL COVID-19 section” was filled out for only 34 MHD patients. Finally, the molecular swab did not confirm the nasopharyngeal swab for 21 (24%) MHD patients, and nephrologists could not provide information for six (7%) patients (Figure 1).
Figure 1.
Flowchart to assess the completeness of the “RRDTL COVID-19 section”.
3.2. Infected Patients Considered in the Study
Thus, a total of 256 patients (64.1% male, mean age 68.4 years (STD: 14.1 years)) were identified as having COVID-19. The characteristics of the patients are summarized in Table 1 according to infection status. Italian natives were 88.6%; 48.6% were resident in Rome, 40.2% had more than 8 years of education and 62.2% of MHD patients were totally autonomous.
Table 1.
Demographic and clinical characteristics of hemodialysis patients by infection status.
| Total | Non-Infected | Infected | p-Value χ2 | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Total | 4942 | 4686 | 256 | |||||
| Gender | 0.609 | |||||||
| Male | 3239 | 65.5 | 3075 | 65.6 | 164 | 64.1 | ||
| Female | 1703 | 34.5 | 1611 | 34.4 | 92 | 35.9 | ||
| Age (years) | 0.843 | |||||||
| 18–64 | 1710 | 34.6 | 1618 | 34.5 | 92 | 35.9 | ||
| 65–84 | 2713 | 54.9 | 2577 | 55.0 | 136 | 53.1 | ||
| ≥85 | 519 | 10.5 | 491 | 10.5 | 28 | 10.9 | ||
| Site of birth | 0.001 | |||||||
| Italy | 4379 | 88.6 | 4169 | 89.0 | 210 | 82.0 | ||
| Abroad | 563 | 11.4 | 517 | 11.0 | 46 | 18.0 | ||
| Residence | 0.171 | |||||||
| Rome municipality | 2403 | 48.6 | 2264 | 48.3 | 139 | 54.3 | ||
| Rome province | 1243 | 25.2 | 1187 | 25.3 | 56 | 21.9 | ||
| Other Lazio municipalities | 1296 | 26.2 | 1235 | 26.4 | 61 | 23.8 | ||
| Educational level (years) | 0.049 | |||||||
| Illiterate | 198 | 4.0 | 181 | 3.9 | 17 | 6.6 | ||
| 1–8 | 2757 | 55.8 | 2610 | 55.7 | 147 | 57.4 | ||
| ≥9 | 1987 | 40.2 | 1895 | 40.4 | 92 | 35.9 | ||
| Self-sufficiency | 0.351 | |||||||
| Total autonomy | 3073 | 62.2 | 2919 | 62.3 | 154 | 60.2 | ||
| Autonomy in some activities | 1118 | 22.6 | 1051 | 22.4 | 67 | 26.2 | ||
| Non self-sufficient | 751 | 15.2 | 716 | 15.3 | 35 | 13.7 | ||
| Nephropathy | ||||||||
| Diabetic nephropathy | 969 | 19.6 | 912 | 19.5 | 57 | 22.3 | 0.271 | |
| Renal vascular disease | 915 | 18.5 | 863 | 18.4 | 52 | 20.3 | 0.447 | |
| Glomerulonephritis | 452 | 9.1 | 430 | 9.2 | 22 | 8.6 | 0.753 | |
| Cystic renal disease or familial nephropathy | 389 | 7.9 | 358 | 7.6 | 31 | 12.1 | 0.010 | |
| Interstitial and toxic nephritis/pyelonephritis | 183 | 3.7 | 175 | 3.7 | 8 | 3.1 | 0.615 | |
| Systemic disease | 152 | 3.1 | 143 | 3.1 | 9 | 3.5 | 0.675 | |
| Renal malformation | 52 | 1.1 | 50 | 1.1 | 2 | 0.8 | 0.663 | |
| Other nephropathies | 621 | 12.6 | 596 | 12.7 | 25 | 9.8 | 0.165 | |
| Unknown | 1209 | 24.5 | 1159 | 24.7 | 50 | 19.5 | 0.059 | |
| Comorbidity | ||||||||
| Heart disease | 1711 | 34.6 | 1616 | 34.5 | 95 | 37.1 | 0.390 | |
| Diabetes | 1379 | 27.9 | 1307 | 27.9 | 72 | 28.1 | 0.935 | |
| Severe hypertension | 785 | 15.9 | 737 | 15.7 | 48 | 18.8 | 0.198 | |
| Obesity | 772 | 15.6 | 723 | 15.4 | 49 | 19.1 | 0.111 | |
| Vascular disease | 749 | 15.2 | 724 | 15.5 | 25 | 9.8 | 0.014 | |
| Cerebrovascular disease | 684 | 13.8 | 644 | 13.7 | 40 | 15.6 | 0.396 | |
| Cancer | 670 | 13.6 | 639 | 13.6 | 31 | 12.1 | 0.487 | |
| COPD | 609 | 12.3 | 573 | 12.2 | 36 | 14.1 | 0.385 | |
| Thyroid disease | 607 | 12.3 | 580 | 12.4 | 27 | 10.5 | 0.385 | |
| Lipid metabolism’s alteration | 447 | 9.0 | 427 | 9.1 | 20 | 7.8 | 0.480 | |
| Renal transplant | 400 | 8.1 | 381 | 8.1 | 19 | 7.4 | 0.686 | |
| Malnutrition | 293 | 5.9 | 275 | 5.9 | 18 | 7.0 | 0.443 | |
| Liver disease | 215 | 4.4 | 208 | 4.4 | 7 | 2.7 | 0.193 | |
| Motor deficit | 142 | 2.9 | 134 | 2.9 | 8 | 3.1 | 0.805 | |
| Extra-uremic anemia | 131 | 2.7 | 127 | 2.7 | 4 | 1.6 | 0.266 | |
| IBD | 129 | 2.6 | 119 | 2.5 | 10 | 3.9 | 0.182 | |
| Peptic ulcer | 90 | 1.8 | 86 | 1.8 | 4 | 1.6 | 0.751 | |
| Psychiatric disease | 89 | 1.8 | 81 | 1.7 | 8 | 3.1 | 0.102 | |
| Dementia | 83 | 1.7 | 75 | 1.6 | 8 | 3.1 | 0.065 | |
| LHA of dialysis centre | 0.014 | |||||||
| Rome 1 | 967 | 19.6 | 920 | 19.6 | 47 | 18.4 | ||
| Rome 2 | 1026 | 20.8 | 949 | 20.3 | 77 | 30.1 | ||
| Rome 3 | 489 | 9.9 | 468 | 10.0 | 21 | 8.2 | ||
| Rome 4 | 275 | 5.6 | 262 | 5.6 | 13 | 5.1 | ||
| Rome 5 | 425 | 8.6 | 403 | 8.6 | 22 | 8.6 | ||
| Rome 6 | 523 | 10.6 | 505 | 10.8 | 18 | 7.0 | ||
| Viterbo | 199 | 4.0 | 193 | 4.1 | 6 | 2.3 | ||
| Latina | 88 | 1.8 | 86 | 1.8 | 2 | 0.8 | ||
| Rieti | 501 | 10.1 | 479 | 10.2 | 22 | 8.6 | ||
| Frosinone | 449 | 9.1 | 421 | 9.0 | 28 | 10.9 | ||
| Weekly hours of dialysis | 0.104 | |||||||
| 3–9 | 1008 | 20.4 | 966 | 20.6 | 42 | 16.4 | ||
| ≥10 | 3934 | 79.6 | 3720 | 79.4 | 214 | 83.6 | ||
| Hemodialysis vintage (years) | 0.214 | |||||||
| 0–1 | 1900 | 38.4 | 1811 | 38.6 | 89 | 34.8 | ||
| ≥2 | 3042 | 61.6 | 2875 | 61.4 | 167 | 65.2 | ||
The most common cause of ESKD was diabetic nephropathy (19.6%), while heart disease and diabetes were the comorbidities (34.6% and 27.9%, respectively) with the highest prevalence. Half the patients were followed by a dialysis unit located in a LHA of Rome. Most of the patients (61.6%) had been on dialysis for 2 years or more; 80% of patients underwent dialysis treatment for more than 10 h a week.
A statistically significant difference (p-value < 0.05) in the distribution of covariates between non-infected and infected MHD patients was observed. Italian natives were, respectively, 89% and 82% in non-infected and infected patients. Illiteracy was highest among the infected (6.6% vs. 3.9%, respectively). Having cystic renal disease or familial nephropathy was more frequent among the infected MHD patients (12.1% vs. 7.6%, respectively), while having vascular disease was less common among the infected. Finally, a statistically significant difference was also detected in the LHA of the dialysis centers among infected/non-infected patients (Table 1).
3.3. Risk of SARS-CoV-2 Infection among Hemodialysis Patients and Main Risk Factors
The crude risk of SARS-CoV-2 infection was 5.18% (95% CI 4.58–5.85), while the age- and gender-adjusted risk was 5.05% (95% CI 4.34–5.89).
Variables significantly associated with the outcome at the univariate level were as follows: place of birth, cystic renal disease or familial nephropathy, vascular disease and LHA of hemodialysis center (Table 1).
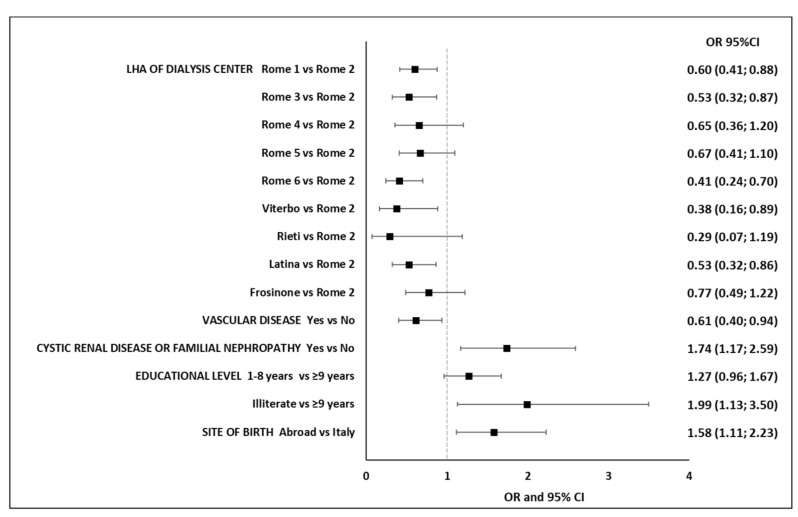
The multivariable logistic regression model showed that place of birth other than Italy (OR 1.58, 95% CI 1.11–2.23), being illiterate vs. ≥9 years of education (OR 1.99, 95% CI 1.13–3.50) and cystic renal disease or familial nephropathy (OR 1.74, 95% CI 1.17–2.59) had a statistically significant association with an increased risk of infection, while vascular disease (OR 0.61, 95% CI 0.40–0.94) and being treated in dialysis centers belonging to an LHA other than Roma 2 (ORs less than 1) proved to be protective (Figure 2). Moreover, age group and gender were not risk factors for SARS-CoV-2 infection among MHD patients.
Figure 2.
Factors associated with SARS-CoV-2 infection among hemodialysis patients.
3.4. 30-Day Mortality from Infection Onset and Main Risk Factors
Of the 256 SARS-CoV-2-infected MHD patients, 59 patients (23%) died within 30 days (64.4% males, mean age 74.4 years; STD: 11.7 years). The patient characteristics according to vital status are shown in Table 2.
Table 2.
Characteristics of hemodialysis patients infected with SARS-CoV-2 by vital status within 30 days of infection onset.
| Total | Alive | Dead | p-Value χ2 | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Total | 256 | 197 | 59 | |||||
| Period of infection incidence | 0.006 | |||||||
| March-July | 37 | 14.5 | 22 | 11.2 | 15 | 25.4 | ||
| August-November | 219 | 85.5 | 175 | 88.8 | 44 | 74.6 | ||
| Gender | 0.950 | |||||||
| Male | 164 | 64.1 | 126 | 64.0 | 38 | 64.4 | ||
| Female | 92 | 35.9 | 71 | 36.0 | 21 | 35.6 | ||
| Age (years) | 0.005 | |||||||
| 18–64 | 90 | 35.2 | 76 | 38.6 | 14 | 23.7 | ||
| 65–84 | 134 | 52.3 | 103 | 52.3 | 31 | 52.5 | ||
| ≥85 | 32 | 12.5 | 18 | 9.1 | 14 | 23.7 | ||
| Site of birth | 0.075 | |||||||
| Italy | 210 | 82.0 | 157 | 79.7 | 53 | 89.8 | ||
| Abroad | 46 | 18.0 | 40 | 20.3 | 6 | 10.2 | ||
| Residence | 0.114 | |||||||
| Rome municipality | 139 | 54.3 | 100 | 50.8 | 39 | 66.1 | ||
| Rome province | 56 | 21.9 | 46 | 23.4 | 10 | 16.9 | ||
| Other Lazio municipalities | 61 | 23.8 | 51 | 25.9 | 10 | 16.9 | ||
| Educational level (years) | 0.622 | |||||||
| Illiterate | 17 | 6.6 | 14 | 7.1 | 3 | 5.1 | ||
| 1–8 | 147 | 57.4 | 110 | 55.8 | 37 | 62.7 | ||
| ≥9 | 92 | 35.9 | 73 | 37.1 | 19 | 32.2 | ||
| Self-sufficiency | 0.705 | |||||||
| Total autonomy | 154 | 60.2 | 120 | 60.9 | 34 | 57.6 | ||
| Autonomy in some activities | 67 | 26.2 | 52 | 26.4 | 15 | 25.4 | ||
| Non self-sufficient | 35 | 13.7 | 25 | 12.7 | 10 | 16.9 | ||
| Nephropathy | ||||||||
| Diabetic nephropathy | 57 | 22.3 | 48 | 24.4 | 9 | 15.3 | 0.140 | |
| Renal vascular disease | 52 | 20.3 | 38 | 19.3 | 14 | 23.7 | 0.457 | |
| Cystic renal disease or familial nephropathy | 31 | 12.1 | 22 | 11.2 | 9 | 15.3 | 0.399 | |
| Glomerulonephritis | 22 | 8.6 | 16 | 8.1 | 6 | 10.2 | 0.623 | |
| Systemic disease | 9 | 3.5 | 8 | 4.1 | 1 | 1.7 | 0.387 | |
| Interstitial and toxic nephritis/pyelonephritis | 8 | 3.1 | 4 | 2.0 | 4 | 6.8 | 0.066 | |
| Renal malformation | 2 | 0.8 | 1 | 0.5 | 1 | 1.7 | 0.364 | |
| Other nephropathies | 25 | 9.8 | 18 | 9.1 | 7 | 11.9 | 0.536 | |
| Unknown | 52 | 20.3 | 42 | 21.3 | 8 | 13.6 | 0.187 | |
| Comorbidity | ||||||||
| Heart disease | 95 | 37.1 | 72 | 36.5 | 23 | 39.0 | 0.734 | |
| Diabetes | 72 | 28.1 | 57 | 28.9 | 15 | 25.4 | 0.599 | |
| Obesity | 49 | 19.1 | 39 | 19.8 | 10 | 16.9 | 0.626 | |
| Severe hypertension | 48 | 18.8 | 43 | 21.8 | 5 | 8.5 | 0.021 | |
| Cerebrovascular disease | 40 | 15.6 | 27 | 13.7 | 13 | 22.0 | 0.122 | |
| COPD | 36 | 14.1 | 26 | 13.2 | 10 | 16.9 | 0.467 | |
| Cancer | 31 | 12.1 | 23 | 11.7 | 8 | 13.6 | 0.697 | |
| Thyroid disease | 27 | 10.5 | 16 | 8.1 | 11 | 18.6 | 0.021 | |
| Vascular disease | 25 | 9.8 | 16 | 8.1 | 9 | 15.3 | 0.106 | |
| Lipid metabolism’s alteration | 20 | 7.8 | 16 | 8.1 | 4 | 6.8 | 0.736 | |
| Renal transplant | 19 | 7.4 | 14 | 7.1 | 5 | 8.5 | 0.725 | |
| Malnutrition | 18 | 7.0 | 8 | 4.1 | 10 | 16.9 | 0.001 | |
| IBD | 10 | 3.9 | 6 | 3.0 | 4 | 6.8 | 0.194 | |
| Motor deficit | 8 | 3.1 | 5 | 2.5 | 3 | 5.1 | 0.324 | |
| Dementia | 8 | 3.1 | 5 | 2.5 | 3 | 5.1 | 0.324 | |
| Psychiatric disease | 8 | 3.1 | 6 | 3.0 | 2 | 3.4 | 0.894 | |
| Liver disease | 7 | 2.7 | 4 | 2.0 | 3 | 5.1 | 0.207 | |
| Extra-uremic anemia | 4 | 1.6 | 3 | 1.5 | 1 | 1.7 | 0.926 | |
| Peptic ulcer | 4 | 1.6 | 4 | 2.0 | 0 | 0.0 | 0.270 | |
| LHA of dialysis centre | 0.033 | |||||||
| Rome 1 | 47 | 18.4 | 35 | 17.8 | 12 | 20.3 | ||
| Rome 2 | 77 | 30.1 | 58 | 29.4 | 19 | 32.2 | ||
| Rome 3 | 21 | 8.2 | 15 | 7.6 | 6 | 10.2 | ||
| Rome 4 | 13 | 5.1 | 9 | 4.6 | 4 | 6.8 | ||
| Rome 5 | 22 | 8.6 | 20 | 10.2 | 2 | 3.4 | ||
| Rome 6 | 18 | 7.0 | 11 | 5.6 | 7 | 11.9 | ||
| Viterbo | 6 | 2.3 | 4 | 2.0 | 2 | 3.4 | ||
| Latina | 2 | 0.8 | 0 | 0.0 | 2 | 3.4 | ||
| Rieti | 22 | 8.6 | 19 | 9.6 | 3 | 5.1 | ||
| Frosinone | 28 | 10.9 | 26 | 13.2 | 2 | 3.4 | ||
| Weekly hours of dialysis | 0.501 | |||||||
| 3–9 | 42 | 16.4 | 34 | 17.3 | 8 | 13.6 | ||
| ≥10 | 214 | 83.6 | 163 | 82.7 | 51 | 86.4 | ||
| Hemodialysis vintage (years) | 0.155 | |||||||
| 0–1 | 80 | 31.3 | 66 | 33.5 | 14 | 23.7 | ||
| ≥2 | 176 | 68.8 | 131 | 66.5 | 45 | 76.3 | ||
| Symptoms at time of swab | ||||||||
| Fever | 141 | 55.1 | 100 | 50.8 | 41 | 69.5 | 0.011 | |
| Cough | 83 | 32.4 | 61 | 31.0 | 22 | 37.3 | 0.363 | |
| Respiratory distress | 52 | 20.3 | 34 | 17.3 | 18 | 30.5 | 0.027 | |
| Colds | 11 | 4.3 | 9 | 4.6 | 2 | 3.4 | 0.695 | |
| Gastrointestinal disease | 7 | 2.7 | 5 | 2.5 | 2 | 3.4 | 0.725 | |
| Conjunctivitis | 5 | 2.0 | 4 | 2.0 | 1 | 1.7 | 0.870 | |
| Loss of taste and smell | 2 | 0.8 | 2 | 1.0 | 0 | 0.0 | 0.437 | |
| Hospitalization for infection | 0.549 | |||||||
| No | 64 | 25.0 | 51 | 25.9 | 13 | 22.0 | ||
| Yes | 192 | 75.0 | 146 | 74.1 | 46 | 78.0 | ||
In the period August–November 2020, 85.5% of infections were recorded. The most common symptoms at the time of swab were fever (55.1%), cough (32.4%) and respiratory distress (20.3%); 75.0% of MHD patients infected by SARS-CoV-2 were hospitalized.
Statistically significant differences (p-value < 0.05) by vital status in MHD patients were found for: period of infection incidence (March–July: 25.4% in dead vs. 11.2% in alive patients), age (≥85 years: 23.7% in dead and 9.1% in alive patients), having thyroid disorder, being malnourished and having fever or respiratory distress at the time of first swab were more frequent in dead then in alive patients. The prevalence of severe hypertension was not a mortality risk factor (8.5% vs. 21.8%, dead vs. alive, respectively). Statistically significant differences in the distribution of dialysis centers’ LHA between alive/dead patients were found (Table 2).
The crude risk of 30-day mortality was 23.1% (95% CI 17.9–29.8), while the age- and gender-adjusted risk was 21.0% (95% CI 15.0–29.6).
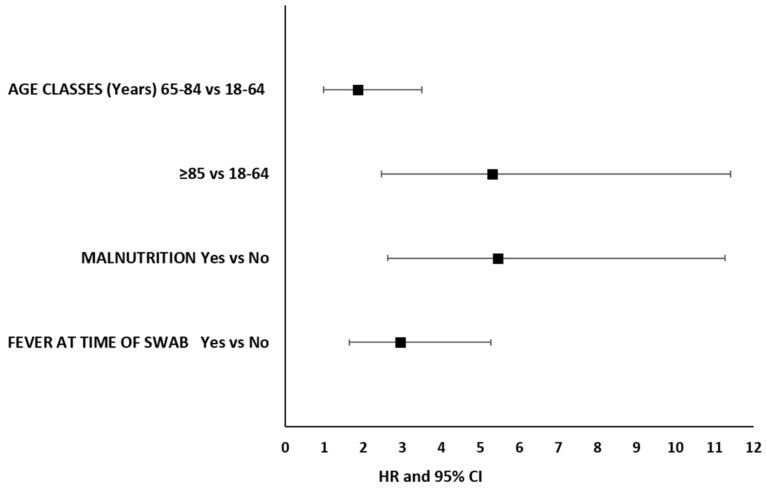
Factors associated with 30-day mortality were being 85 years old or more (HR 5.31, 95% CI 2.47–11.42 ≥ 85 vs. 18–64 years), being malnourished (HR 5.44, 95% CI 2.62–11.28) and having a fever at the time of swab (HR 2.95, 95% CI 1.65–5.27) (Figure 3). Protective factors were not identified.
Figure 3.
Factors associated with 30-day mortality in hemodialysis patients infected with SARS-CoV-2.
4. Discussion
End-stage kidney disease patients on MHD represent a high-risk subgroup for SARS-CoV-2 infection due to the higher probability of cross-contamination in closed environments and to the impairment of both adaptive and innate immunity related to their uremic state [50,51]. Moreover, the presence of multiple comorbidities (e.g., hypertension, cardiovascular disease, diabetes mellitus) in this population may be associated with a higher risk of adverse outcomes [50,51].
Our population-based cohort study provides information on the impact of SARS-CoV-2 among MHD patients in a large Italian region during the year 2020. Both the risk of infection (5.1%) and of mortality (21.0%) are high, confirming the greater vulnerability of this population and a worse outcome if compared with that reported in the general population [52].
In a previous study performed on the same population, during the period 1 March–31 July 2020, it was observed that there was a mortality risk of 5.5% higher in the dialyzed population with COVID-19 compared with the uninfected dialyzed population [53].
Regarding the impact of COVID-19 on the MHD population, a national survey conducted by the Italian Society of Nephrology during the first wave of the COVID-19 pandemic (April 2020), which considered data from 80.4% of Italian nephrology centers, reported a proportion of 3.55% of MHD patients to be positive for SARS-CoV-2 [54]. Such prevalence is lower than that reported here, and the difference could derive from the heterogeneity in the intensity of pandemic spread by region in Italy [55]; indeed, the highest proportion of infected patients was reported in northern Italy, probably as a consequence of the rapid growth of infected cases in the general population of that area [16], while a much lower proportion was detected in southern Italy, resulting in a lower average incidence in the country. For example, in one of the first regions to be hit by the pandemic, with dramatically high incidence rates, the Brescia Renal COVID Task Force reported SARS-CoV-2 RNA positivity in 94 out of 643 MHD patients (15%) [16]. Furthermore, the higher incidence of SARS-CoV-2 infection among MHD patients compared to the general population has been confirmed in several countries, independently from the phase of the pandemic [56,57,58]. These findings could be largely explained by some peculiarities of MHD patients, such as contact with healthcare providers 2–3 times a week and potential issues in physical distancing during transportation to dialysis units, in the waiting room and during the treatment session. Furthermore, multiple comorbidities of MHD patients could require additional day-to-day assistance or support which could impede social distancing. Similar to that reported elsewhere [59], our results are suggestive of different incidence of SARS-CoV-2 infection in relation to education level and place of birth. It could be speculated that these findings reflect socioeconomic factors and living conditions which do not favor the compliance of social distancing measures in place [57,58]. Moreover, the finding that the incidence of infection was higher in MHD patients assisted in a particular area of Lazio region (LHA Roma 2) could be partially explained by the documented higher incidence of infection in the general population living in that area [60].
The mortality risk reported in our study is comparable to that reported elsewhere. Indeed, in relation to the heterogeneity of the cohorts of MHD patients included in studies performed in various countries during different phases of the pandemic, the mortality rate appears to range between 20% and 35% [33,54,56,59,61,62,63]. For example, in a multicenter French cohort, including patients on MHD from 11 dialysis centers, a COVID-19 prevalence of 5.5% and a mortality rate of 28% were reported among 122 patients with a follow-up of at least 28 days [58]. Another study conducted in the UK, using the UK Renal Registry, reported a slightly lower mortality rate of 20%, possibly due to the shorter follow-up time period considered: 14 days from SARS-CoV-2 positivity [52].
As reported in previous studies [51,52,63], we found that advanced age and patients’ frailty, mainly malnutrition status, were associated with a higher risk of mortality.
Strengths of this study are the large sample size and the integrated use of several regional databases. These tools are subjected to continuous quality controlling that allow us to have accurate and standardized data; the completeness of the “RRDTL COVID-19 section” confirms the high quality of data collection. On the other hand, a limitation of the study is the lack of information about potential confounding clinical parameters not included in the RRDTL (e.g., inter-current acute illness unrelated to COVID-19 during hospital stay), limiting our capability to exclude other residual bias. Finally, dialysis patients did not have regular nasopharyngeal swabs as a screening tool; hence, it is possible that a minimal number of asymptomatic SARS-CoV-2 infection patients were not diagnosed and were excluded from the study.
5. Conclusions
In conclusion, our registry-based dialysis cohort study, conducted in the Lazio region, confirms that MHD patients represent a vulnerable group at particularly high risk of SARS-CoV-2 infection and severe health outcomes. The risk of infection appears to be higher in the presence of conditions that make difficult the maintenance of adequate social distancing and seems to reflect socioeconomic status and the prevalence of infection in the territory where patients underwent dialysis treatment. It is therefore important to ensure the adherence to the recommendations of the guidelines implemented to mitigate the risk of infection spread in outpatient hemodialysis facilities. It is also necessary to ensure high vaccination coverage in these frail patients. Moreover, presence of fever at the time of swab emerged as a significant 30-day mortality risk factor, indicating the need for constant monitoring and additional care of this subgroup starting from the early phase of infection. The adoption of extended indications for hospitalization in COVID-19 MHD patients was recommended, especially in the presence of fever or need for oxygen therapy at the time of swab.
Acknowledgments
Members of the Regional Dialysis and Transplant Registry (on September 2021): Moreno Aleandri, Carmelo Alfarone, Giuseppe Maria Antonio Aloisio, Ernesto Anselmo Cioffi, Guido Baldinelli, Eligio Boccia, Franco Bondatti, Enrico Bordoni, Francesco Canulla, Antonio Carnabuci, Benedetta Cartenì, Angelo Emanuele Catucci, Maria Grazia Chiappini, Giuseppe Ciano, Carlo Cuzziol, Anna D’Apollo Maria, Paolo De Paolis, Piergianni De Marino, Barbara della Grotta, Rinaldo Di Toro, Luca Di Lullo, Gabriele Di Pietro, Fiorella Faraglia, Loredana Fazzari, Roberto Felicioni, Sandro Feriozzi, Armando Filippini, Gabriele Firmi, Alessandro Flammini, Franco Forte, Marco Gamberini, Fabio Gangeri, Isabella Guzzo, Liliana Kristuli, Raffaella Lavini, Maurizio Lonzi, Marco Marin, Rocco Marinelli, Fulvio Marrocco, Anna Paola Mitterhofer, Augusto Morgia, Massimo Morosetti, Alessandro Naticchia, Livia Nazzaro, Angelo Nigro, Carlo Nusca, Leandro Onorato, Ettore Pala, Roberto Palumbo, Nicola Panocchia, Lucia Pantano, Antonio Paone, Stefania Pizzarelli, Lelio Polidori, Pasquale Polito, Marialaura Puliti, Giorgio Punzo, Costantino Ricci, Nunzio Rifici, Elsa Rizzi, Valeria Rossi, Silverio Rotondi, Luca Scabbia, Monica Serraiocco, Pietro Sfregola, Pergiorgio Simeoni, Marzia Simonelli, Roberto Simonelli, Pierre Elias Soggia, Massimo Spaziani, Giorgio Splendiani, Vittorio Stranges, Maria Teresa Ferrazzano, Maurizio Terra, Maria Cristina Torre, Antonio Treglia, Simonetta Vastano. Members of the Dialysis and Transplant Lazio Region Registry Scientific Committee (on September 2021): Maurizio Bossola, Maria Grazia Chiappini, Ernesto Cioffi, Carmine De Cicco, Paolo De Paolis, Salvatore Di Giulio, Sandro Feriozzi, Pietro Manuel Ferraro, Marco Galliani, Giuseppe Grandaliano, Isabella Guzzo, Fulvio Marrocco, Sandro Mazzaferro, Paolo Mene’, Marco Pignocco, Anna Rachele Rocca, Luigi Tazza, Giuseppe Tisone, Nicola Torlone, Antonio Treglia, Ilaria Umbro, Maurizio Valeri, Elio Vitaliano. Thanks to Francesca de’ Donato for English revision and valuable suggestions.
Author Contributions
Conceptualization, C.M., L.A., S.M., V.P., A.M.B., N.P., N.A. and M.D.; methodology, C.M., L.A., E.C. and S.C.; formal analysis, C.M., L.A. and A.D.N.; data curation, C.M., E.C. and S.C.; writing—original draft, C.M., L.A., S.M. and V.P.; writing—review and editing, C.M., L.A., S.M., V.P., E.C., S.C., A.M.B., N.P., A.D.N., N.A. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Institutional Review Board
Ethics committee or institutional review board approval and informed consent were not necessary because the authors used the data already collected at the beginning of the study, and the data were analyzed anonymously through a standardized methodology according to the Italian national privacy law (national legislative decree on privacy policy no. 196/30 June 2003). The Department of Epidemiology of the Lazio Regional Health Service is the regional referral center for epidemiological research, and it has full access to anonymized health information systems and Regional Dialysis and Transplant Registry.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data related to the findings reported in our manuscript are available to all interested researchers upon reasonable request and with the permission of the Regional Department because of stringent legal restrictions regarding the privacy policy on personal information in Italy (national legislative decree on privacy policy n. 196/30 June 2003). For these reasons our dataset cannot be made available on public data deposition.
Conflicts of Interest
The authors declare that they have no conflicts of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Novel Coronavirus—China. [(accessed on 17 May 2020)]. Available online: https://www.who.int/csr/don/12-january2020-novel-coronavirus-china/en/
- 2.Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019nCoV) [(accessed on 2 May 2020)]. Available online: https://www.who.int/newsroom/detail/30012020statementonthesecondmeetingoftheinternationalhealthregulations(2005)emergencycommitteeregardingtheoutbreakofnovelcoronavirus.
- 5.Johns Hopkins University Coronavirus Resource Centre. Johns Hopkins University. 2020. [(accessed on 1 December 2020)]. Available online: https://coronavirus.jhu.edu/map.html.
- 6.Andamento Della Mortalità Giornaliera (SiSMG) Nelle Città Italiane in Relazione All’epidemia di COVID-19 Rapporto 1 Settembre 2020–6 Aprile 2021. [(accessed on 15 July 2021)]; Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_3047_allegato.pdf.
- 7.Naicker S., Yang C.-W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97:824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARSCoV2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izzedine H., Jhaveri K.D., Perazella M.A. Vascular injury and COVID-19 related mortality: What lies below the tip of the iceberg? Clin. Nephrol. 2020;94:11–13. doi: 10.5414/CN110217. [DOI] [PubMed] [Google Scholar]
- 12.Madjid M., SafaviNaeini P., Solomon S.D., Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., Hazzan A.D., Fishbane S., Jhaveri K.D. Northwell COVID-19 Research Consortium; et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Northwell COVID-19 Research Consortium Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma P., Uppal N.N., Wanchoo R., Shah H.H., Yang Y., Parikh R., Khanin Y., Madireddy V., Larsen C.P., Jhaveri K.D., et al. COVID-19 Associated Kidney Injury: A Case Series of Kidney Biopsy Findings. J. Am. Soc. Nephrol. 2020;31:1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A., Maffei C., Possenti S., Lucca B., Cortinovis R., et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and shortterm outcome of hemodialysis patients with SARSCoV2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betjes M.G. Immune cell dysfunction and inflammation in endstage renal disease. Nat. Rev. Nephrol. 2013;9:25565. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 18.Saran R., Robinson B., Abbott K.C., Agodoa L.Y.C., Bhave N., BraggGresham J., Balkrishnan R., Dietrich X., Eckard A., Eggers P.W., et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2018;71((Suppl. S1)):A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., Zhao M.H., Zuo L., Wang Y., Yu F., Zhang H., Wang H., CKNET Work Group China Kidney Disease Network (CKNET) 2015 Annual Data Report. Kidney Int. Suppl. 2019;9:e1–e81. doi: 10.1016/j.kisu.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NICE: COVID-19 Rapid Guideline: Dialysis Service Delivery, London, National Institute for Health and Care Excellence. 2020. [(accessed on 15 July 2021)]. Available online: https://www.nice.org.uk/guidance/ng160. [PubMed]
- 21.Burgner A., Ikizler T.A., Dwyer J.P. COVID-19 and the Inpatient Dialysis Unit: Managing Resources during Contingency Planning Pre-Crisis. Clin. J. Am. Soc. Nephrol. 2020;15:720722. doi: 10.2215/CJN.03750320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kliger A.S., Silberzweig J. Mitigating Risk of COVID-19 in Dialysis Facilities. Clin. J. Am. Soc. Nephrol. 2020;15:707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basile C., Combe C., Pizzarelli F., Covic A., Davenport A., Kanbay M., Kirmizis D., Schneditz D., Sande F., Mitra S. Recommendations for the prevention, mitigation and containment of the emerging SARSCoV2 (COVID-19) pandemic in haemodialysis centres. Nephrol. Dial. Transplant. 2020;35:737–741. doi: 10.1093/ndt/gfaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su K., Ma Y., Wang Y., Song Y., Lv X., Wei Z., Shi M., Ding G., Shen B., Wang H. How we mitigated and contained the COVID-19 outbreak in a hemodialysis center: Lessons and experience. Infect. Control Hosp. Epidemiol. 2020;41:1240–1242. doi: 10.1017/ice.2020.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rombolà G., Heidempergher M., Pedrini L., Farina M., Aucella F., Messa P., Brunori G. Practical indications for the prevention and management of SARSCoV2 in ambulatory dialysis patients: Lessons from the first phase of the epidemics in Lombardy. J. Nephrol. 2020;33:193–196. doi: 10.1007/s40620-020-00727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annual Data Report USRDS COVID-19 Supplement: Chapter 13. [(accessed on 5 May 2021)]. Available online: https://adr.usrds.org/2020/covid19supplement/1covid19supplement.
- 27.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R., Liao C., He H., Hu C., Wei Z., Hong Z., Zhang C., Liao M., Shui H. COVID-19 in Hemodialysis Patients: A Report of 5 Cases. Am. J. Kidney Dis. 2020;76:141–143. doi: 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrey A.J., Choi G., Hanna R.M., Chang Y., Tantisattamo E., Ivaturi K., Park E., Nguyen L., Wang B., Tonthat S., et al. A Case of Novel Coronavirus Disease 19 in a Chronic Hemodialysis Patient Presenting with Gastroenteritis and Developing Severe Pulmonary Disease. Am. J. Nephrol. 2020;51:337–342. doi: 10.1159/000507417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang B., Li S., Xiong Y., Tian M., Yu J., Xu L., Zhang L., Li Z., Ma J., Wen F., et al. COVID-19 Pneumonia in a Hemodialysis Patient. Kidney Med. 2020;2:354–358. doi: 10.1016/j.xkme.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Shamy O., Sharma S., Winston J., Uribarri J. Peritoneal Dialysis During the Coronavirus Disease2019 (COVID-19) Pandemic: Acute Inpatient and Maintenance Outpatient Experiences. Kidney Med. 2020;2:377–380. doi: 10.1016/j.xkme.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudreuilh C., Kumar N., Moxham V., Hemsley C., Goldenberg S., Moutzouris D.A. Deisolation of COVID-19 positive hemodialysis patients in the outpatient setting: A singlecenter experience. Kidney Int. 2020;98:236–237. doi: 10.1016/j.kint.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valeri A.M., Robbins-Juarez S.Y., Stevens J.S., Ahn W., Rao M.K., Radhakrishnan J., Gharavi A.G., Mohan S., Husain S.A. Presentation and Outcomes of Patients with ESKD and COVID-19. J. Am. Soc. Nephrol. 2020;31:1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng J.H., Hirsch J.S., Wanchoo R., Sachdeva M., Sakhiya V., Hong S., Jhaveri K.D., Fishbane S. Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98:1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Y., Diao B., Lv X., Zhu J., Liang W., Liu L., Bu W., Cheng H., Zhang S., Shi M., et al. Novel Coronavirus Disease in Hemodialysis (HD) Patients: Report from One HD Centre in Wuhan China. medRxiv. 2019 doi: 10.1101/2020.02.24.20027201. (submitted) [DOI] [Google Scholar]
- 36.Rombolà G., Brunini F. COVID-19 and dialysis: Why we should be worried. J. Nephrol. 2020;33:401–403. doi: 10.1007/s40620-020-00737-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah A.D., Calabro-Kailukaitis N. COVID-19 and ESKD, A Rapid Review. Rhode Isl. Med. J. 2020;103:29–33. [PubMed] [Google Scholar]
- 38.Sánchez-Álvarez J.E., Fontán M.P., Martín C.J., Pelícano M.B., Reina C.J.C., Prieto Á.M.S., Melilli E., Barrios M.C., Heras M.M., Pino M.D. Status of SARS-CoV-2 Infection in Patients on Renal Replacement Therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Nefrología. 2020;40:272–278. doi: 10.1016/j.nefro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couchoud C., Bayer F., Ayav C., Béchade C., Brunet P., Chantrel F., Frimat L., Galland R., Hourmant M., Laurain E., et al. Low Incidence of SARS-CoV-2, Risk Factors of Mortality and the Course of Illness in the French National Cohort of Dialysis Patients. Kidney Int. 2020;98:1519–1529. doi: 10.1016/j.kint.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palumbo R., Morosetti M., Foniciello M., Ribaldi S., Botti R., Spiga G. Emergenza COVID-19. Percorso per la presa in Carico dei Pazienti con Malattia Renale Cronica. [(accessed on 10 October 2020)]. Available online: https://www.anedonlus.it/wpcontent/uploads/2020/11/REGLAZIO.REGISTROUFFICIALE.2020.0937837.pdf.
- 41.Dipartimento di Epidemiologia del Servizio Sanitario Regionale Regione Lazio; Rilevazione straordinaria COVID-19. 2020. [(accessed on 15 February 2021)]. Available online: http://www.deplazio.net/images/stories/files/RRDTL/manualerrdtlcovid19.pdf.
- 42.Marino C., Pignocco M., Calandrini E., Dei Bardi L., Angelici L., Bargagli A.M., Cascini S., Agabiti N., Davoli M. Registro Dialisi e Trapianto Lazio Rapporto annuale e rilevazione straordinaria COVID19. [(accessed on 15 May 2021)]. Available online: http://www.deplazio.net/images/stories/files/RRDTL/rapporto_rrdtl2020_def_20210628.pdf.
- 43.Tazza L., Angelici L., Marino C., Di Napoli A., Bossola M., De Cicco C., Davoli M., Agabiti N. Determinants of Venous Catheter Hemodialysis Onset and Subsequent Switch to Arteriovenous Fistula: An Epidemiological Study in Lazio Region. J. Vasc. Access. 2021;22:749–758. doi: 10.1177/1129729820959942. [DOI] [PubMed] [Google Scholar]
- 44.Bossola M., Marino C., Di Napoli A., Agabiti N., Tazza L., Davoli M., On behalf of the Dialysis and Transplant Lazio Region Registry Scientific Committee Functional impairment and risk of mortality in patients on chronic hemodialysis: Results of the Lazio Dialysis Registry. J. Nephrol. 2018;31:593–602. doi: 10.1007/s40620-018-0484-4. [DOI] [PubMed] [Google Scholar]
- 45.Tazza L., Di Napoli A., Bossola M., Valle S., Pezzotti P., Luciani G., Di Lallo D., on behalf of Lazio Dialysis Registry Ageing of Patients on Chronic Dialysis: Effects on Mortality-A 12-Year Study. Nephrol. Dial. Transplant. 2008;24:940–947. doi: 10.1093/ndt/gfn575. [DOI] [PubMed] [Google Scholar]
- 46.Di Napoli A., Lapucci E., Baglio G., Di Giulio S. Lazio dialysis registry: Natives vs foreigners. G. Ital. Nefrol. 2015;32 [PubMed] [Google Scholar]
- 47.Di Napoli A., Tazza L., Chicca S., Lapucci E., Silvestri P., Di Lallo D., Michelozzi P., Davoli M. Survival among chronic hemodialysis patients for each type of first vascular access; Proceedings of the 51st Era-Edta Congress; Amsterdam, The Netherlands. 31 May–3 June 2014; p. iii246. [DOI] [Google Scholar]
- 48.Guidelines and procedures for the implementation of a new Mortality Information System (SIM), and the Institution at the Local Health Units of the Nominative Causes of Death Registry (Rencam) in the Lazio Region. (Resolution of the Regional Council n.4302 3 July 1984). Official Bulletin of the Lazio Region n.23. 1984. [(accessed on 15 May 2021)]. [Iin Italian] Available online: https://www.regione.lazio.it/bur.
- 49.Further Urgent Civil Protection Interventions in Relation to the Emergency Related to the Health Risk Related to the Onset of Diseases Arising from Communicable Viral Agents. (Order of Civil Protection Department Presidency of the Council of Ministers no. 640 27 February 2020). Official Journal Serie Generale n.50. [(accessed on 20 April 2021)];2020 [In Italian] Available online: https://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=73469.
- 50.Kooman J.P., van der Sande F.M. COVID-19 in ESRD and Acute Kidney Injury. Blood Purif. 2021;50:610–620. doi: 10.1159/000513214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savino M., Casula A., Santhakumaran S., Pitcher D., Wong E., Magadi W., Evans K.M., Benoy-Deeney F., Griffin J., Plumb L., et al. Sociodemographic Features and Mortality of Individuals on Haemodialysis Treatment Who Test Positive for SARS-CoV-2: A UK Renal Registry Data Analysis. PLoS ONE. 2020;15:e0241263. doi: 10.1371/journal.pone.0241263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyerowitz-Katz G., Merone L. A Systematic Review and Meta-Analysis of Published Research Data on COVID-19 Infection Fatality Rates. Int. J. Infect. Dis. 2020;101:138–148. doi: 10.1016/j.ijid.2020.09.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marino C., Angelici L., Calandrini E., Cascini S., Bargagli A.M., Morabito S., Petrosillo N., Agabiti N., Davoli M. Incidenza ed esiti dell’infezione da SARS-CoV-2 nella popolazione in trattamento dialitico nel Lazio. XLIV Convegno AIE—2020. [(accessed on 29 November 2021)]. Available online: https://www.epidemiologia.it/wp-content/uploads/2020/09/Libro-degli-abstract.pdf.
- 54.Quintaliani G., Reboldi G., Di Napoli A., Nordio M., Limido A., Aucella F., Messa P., Brunori G., Italian Society of Nephrology COVID-19 Research Group Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: Survey of the Italian Society of Nephrology. J. Nephrol. 2020;33:725–736. doi: 10.1007/s40620-020-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nordio M., Reboldi G., Di Napoli A., Quintaliani G., Alberici F., Postorino M., Aucella F., Messa P., Brunori G., Italian Society of Nephrology COVID-19 Research Group Risk factors and action thresholds for the novel coronavirus pandemic. Insights from the Italian Society of Nephrology COVID-19 Survey. J. Nephrol. 2021;34:325–335. doi: 10.1007/s40620-020-00946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keller N., Chantrel F., Krummel T., Bazin-Kara D., Faller A.L., Muller C., Nussbaumer T., Ismer M., Benmoussa A., Brahim-Bouna M., et al. Impact of First-Wave COronaVIrus Disease 2019 Infection in Patients on HaemoDIALysis in Alsace: The Observational COVIDIAL Study. Nephrol. Dial. Transplant. 2020;35:1338–1411. doi: 10.1093/ndt/gfaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu C.M., Weiner D.E., Aweh G., Miskulin D.C., Manley H.J., Stewart C., Ladik V., Hosford J., Lacson E.C., Johnson D.S., et al. COVID-19 among US Dialysis Patients: Risk Factors and Outcomes from a National Dialysis Provider. Am. J. Kidney Dis. 2021;77:748–756.e1. doi: 10.1053/j.ajkd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lano G., Braconnier A., Bataille S., Cavaille G., Moussi-Frances J., Gondouin B., Bindi P., Nakhla M., Mansour J., Halin P., et al. Risk Factors for Severity of COVID-19 in Chronic Dialysis Patients from a Multicentre French Cohort. Clin. Kidney J. 2020;13:878–888. doi: 10.1093/ckj/sfaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taji L., Thomas D., Oliver M.J., Ip J., Tang Y., Yeung A., Cooper R., House A.A., McFarlane P., Blake P.G. COVID-19 in Patients Undergoing Long-Term Dialysis in Ontario. CMAJ. 2021;193:E278–E284. doi: 10.1503/cmaj.202601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Department of Epidemiology Lazio Regional Health Service ASL Roma 1 (DEPLAZIO) and Regional Service for Epidemiology, Surveillance and Control of Infectious Diseases INMI Lazzaro Spallanzani (SERESMI) Maps of Lazio. Crude Rates of Cumulative Incidence of Cases COVID19 per Municipality of Residence. [(accessed on 10 October 2020)]. Available online: https://www.dep.lazio.it/covid/covid_map.php.
- 61.Goicoechea M., Cámara L.A.S., Macías N., Muñoz de Morales A., Rojas Á.G., Bascuñana A., Arroyo D., Vega A., Abad S., Verde E., et al. COVID-19: Clinical Course and Outcomes of 36 Hemodialysis Patients in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinhandl E.D., Wetmore J.B., Peng Y., Liu J., Gilbertson D.T., Johansen K.L. Initial Effects of COVID-19 on Patients with ESKD. J. Am. Soc. Nephrol. 2021;32:1444–1453. doi: 10.1681/ASN.2021010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jager K.J., Kramer A., Chesnaye N.C., Couchoud C., Sánchez-Álvarez J.E., Garneata L., Collart F., Hemmelder M.H., Ambühl P., Kerschbaum J., et al. Results from the ERA-EDTA Registry Indicate a High Mortality Due to COVID-19 in Dialysis Patients and Kidney Transplant Recipients across Europe. Kidney Int. 2020;98:1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to the findings reported in our manuscript are available to all interested researchers upon reasonable request and with the permission of the Regional Department because of stringent legal restrictions regarding the privacy policy on personal information in Italy (national legislative decree on privacy policy n. 196/30 June 2003). For these reasons our dataset cannot be made available on public data deposition.