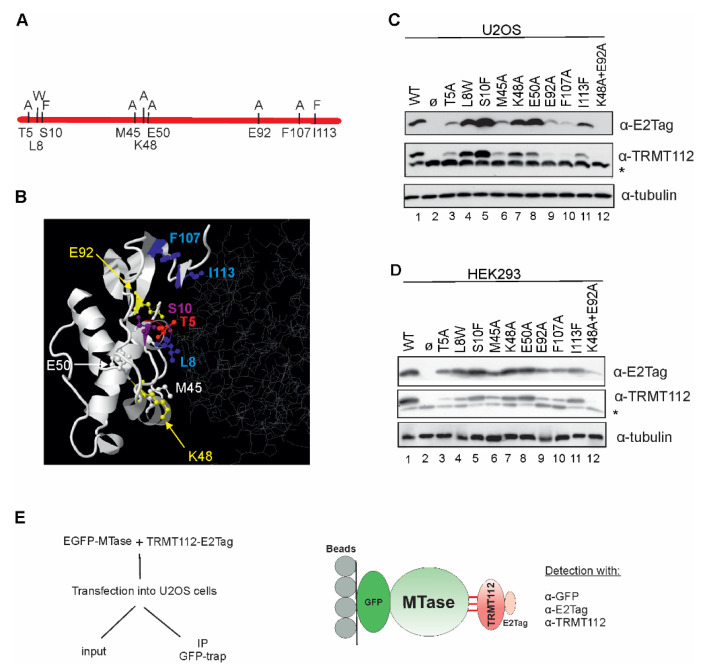
Figure 4.
TRMT112 expression and stability is affected by single amino acid mutations. (A) Schematic view of point-mutations made in this study. Original amino acids are shown under the line and mutations on the line. (B) Localisation of point-mutations on the crystal structure of METTL5-TRMT112 complex (PDB code 6H2U). The structure is visualised with Jmol (http://www.jmol.org/; 08/2021), where TRMT112 is depicted in white ribbon mode and METTL5 in grey wireframe. Mutated amino acids are shown as coloured ball and stick structures. (C) U2OS and (D) HEK293 cells were transfected with plasmids encoding for E2Tagged WT TRMT112 (line 1) and TRMT112 containing single amino acid mutations (lanes 3–12) and an empty vector (lane 2). Cells were analysed 48 h later using antibodies against E2Tag, TRMT112 and tubulin. Images of results with anti-TRMT112 depict both recombinant (upper band) and endogenous (lower band marked with asterisk *) protein. (E) Experimental scheme of the co-immunoprecipitation assay used in this study.