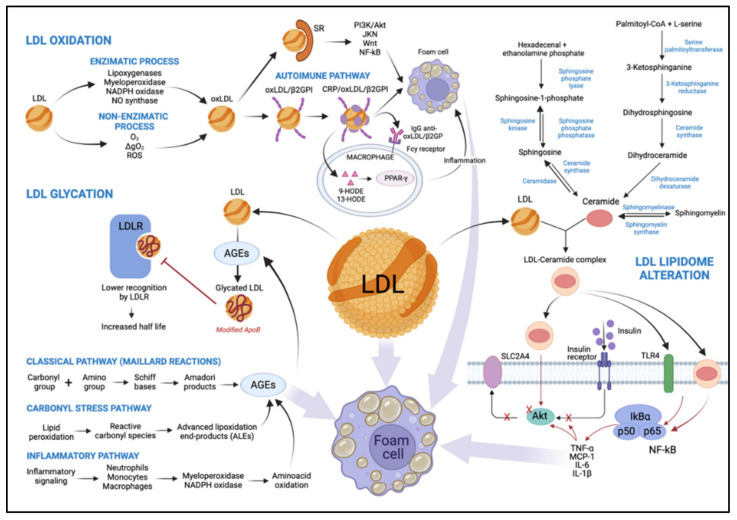
Figure 2.
Representation of the main mechanisms that lead to LDL modification as a result of T2DM. The oxidation process can occur via two main pathways, by an enzymatic or non-enzymatic process, playing an important role in the development of atherosclerosis. Oxidation by the enzymatic process involves lipoxygenases, myeloperoxidases, NADPH oxidase, and nitric oxide synthase. Oxidation by a non-enzymatic process involves free transition metal ions. The Ox-LDL loses its affinity with LDLR and binds to SRs activating signaling pathways like Akt, JNK, Wnt, and NF-κB. It is also capable of forming a complex with β2GPI and CRP, promoting an inflammatory process. It serves as a potent regulator of macrophage gene expression involving PPAR-γ activation through 9-HODE and 13-HODE metabolites. Both mechanisms alter LDL, increasing macrophage uptake, promoting foam cell formation, and leading to more LDL modification. A higher level of AGEs is a consequence of diabetes, and it can be formed by three distinct pathways, both of which decrease affinity with LDLR, promoting an increase in half-life and contributing to the formation of foam cells. Lipidomic alteration of LDL being enriched with ceramides plays an important role in the development of tissue insulin resistance, involving insulin-signaling pathways such as IRS and Akt. They also promote the induction of transcription factors involving inflammation and the consequent formation of foam cells. O3: ozone, 1ΔgO2: singlet oxygen, ROS: reactive oxygen species, SR: scavenger receptors, Ox-LDL: oxidized LDL, β2GPI: beta2-glycoprotein I, CRP: C-reactive protein, LDLR: LDL receptor, 9-HODE and 13-HODE: 9- and 13-hydroxyoctadecadienoic acid, respectively, AGEs: advanced glycation end products, SLC2A4: insulin-sensitive solute carrier family 2, member 4, TLR4: toll-like receptor 4, MCP-1: monocyte chemoattractant protein-1, TNF- α: tumor necrosis factor-α, IL-6 and IL-1β: interleukin 6 and 1β, respectively.