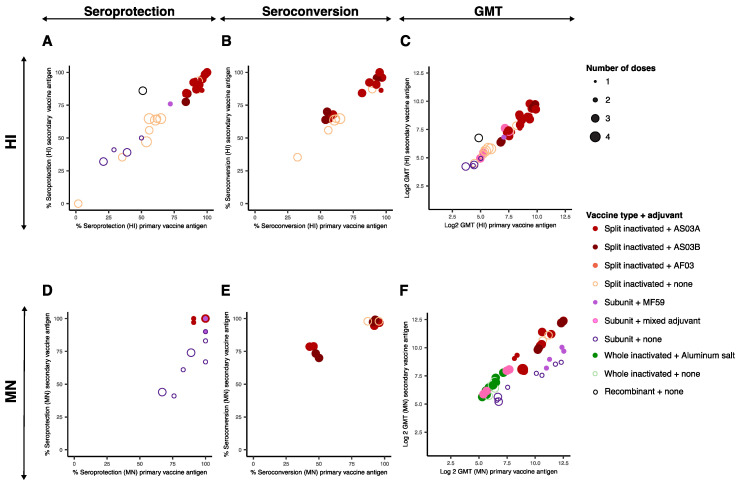
Figure 4.
Immunological endpoints of heterologous vaccination strategies. Immunological endpoint values obtained against the antigen homologous to the primary vaccine strain (x-axis) are plotted against those obtained against the antigen homologous to the secondary vaccine strain (y-axis). Since the vast majority of experimental groups received heterologous vaccination(s) with the same vaccine type as the primary vaccination, the color and fill of the individual points indicate the vaccine type and adjuvant combination used in the primary vaccination according to the legend. The size of the circle indicates the number of vaccine doses (one to four) used before the serum sample was obtained as indicated on the right side of the figure. Data on persistence of the immune response was excluded. (A) Percentage seroprotected subjects measured by HI assay; (B) percentage of seroconverted subjects measured by HI assay; (C) Log2 of the geometric mean HI titer; (D) percentage of seroprotected subjects measured by MN assay; (E) percentage of seroconverted subjects measured by MN assay; (F) Log2 of the geometric mean MN titer. Abbreviations: HI: hemagglutination inhibition; MN: microneutralization; GMT: geometric mean titer.