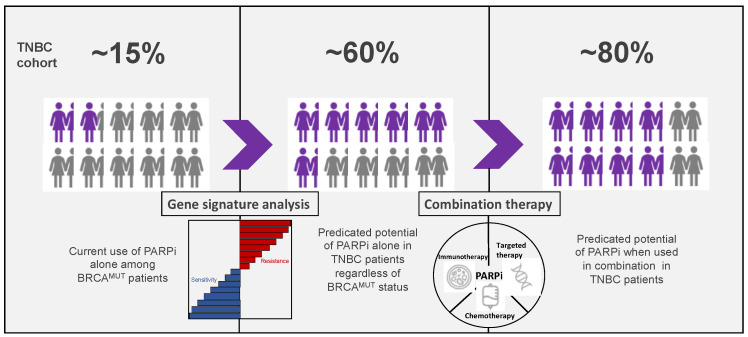
Figure 2.
Projected utility of PARPi in TNBC. Currently, PARPi are used only among germline, BRCA1/2-mutant metastatic TNBC patients, which constitute about 15% of TNBC patients. With the use of strong predictive biomarkers in early TNBC, it is plausible that PARPi sensitivity will be observed in about 60% of TNBC patients (middle panel). In combination with either chemotherapy, immunotherapy, or targeted therapeutics, PARPi sensitivity can potentially increase to 80% of TNBC patients (right panel).