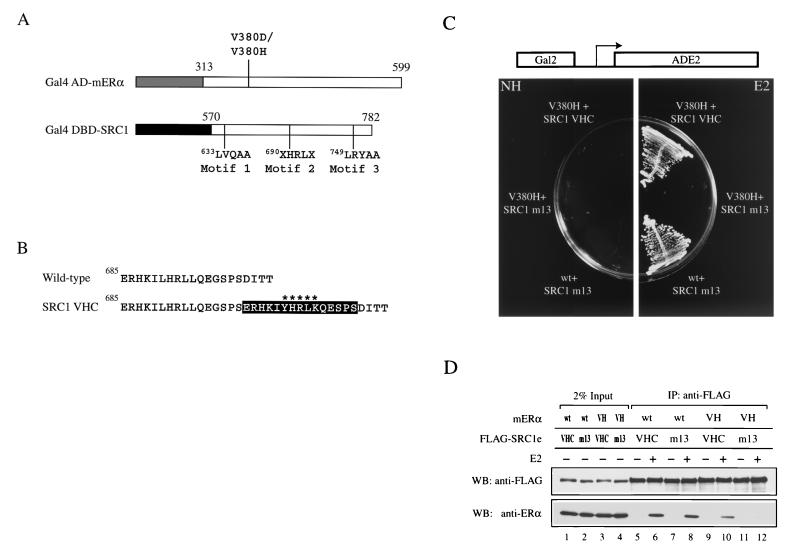
FIG. 2.
Altered-specificity SRC1e. (A) Schematic representation of constructs used in the yeast two-hybrid screen for SRC1 mutants which suppress mutations in V380 of mERα. The numbers indicate amino acid positions in the full-length protein. The letter X represents any amino acids and signifies the two randomized positions. In addition, LXXLL motifs 1 and 3 of SRC1 were rendered nonfunctional by mutation to LXXAA and the construct was denoted by the suffix m13. The lightly shaded box represents the AD of Gal4 (amino acids 768 to 881), and the darkly shaded box represents the DBD of Gal4 (amino acids 1 to 147). (B) Sequence comparison of wild-type SRC1 and SRC1 VHC. The black box encompasses the 15-amino-acid insertion found immediately C terminal to the wild-type LXXLL motif. The variant motif YXXLK is marked with asterisks. (C) Ligand-dependent interaction of mERα V380H with SRC1 VHC in vivo. Expression of Gal4 AD-V380H and Gal4 DBD-SRC1 VHC two-hybrid proteins conferred E2 (100 nM)-dependent growth of yeast strain PJ69-4A on synthetic medium lacking adenine by activating the ADE2 gene, which was under the control of the Gal2 promoter. PJ69-4A transformed with plasmids encoding Gal4 AD-wild type (wt) mERα and Gal4 DBD-SRC1 m13 acted as a positive control. Plates were incubated at 30°C for 2 days. NH, no hormone. (D) Ligand-dependent interaction of mERα V380H with SRC1e VHC in vitro. Full-length mERα and FLAG epitope-tagged wild-type or mutant SRC1e was transiently expressed in 293-T cells, and the whole-cell lysate was subjected to immunoprecipitation (IP) with an anti-FLAG antibody immobilized on agarose beads in the absence or presence of 1 μM E2. SRC1e was detected by Western blotting using an anti-FLAG antibody. The coimmunoprecipitated mERα was detected by using anti-ERα antibody H222. VH, V380H; WB, Western blot.