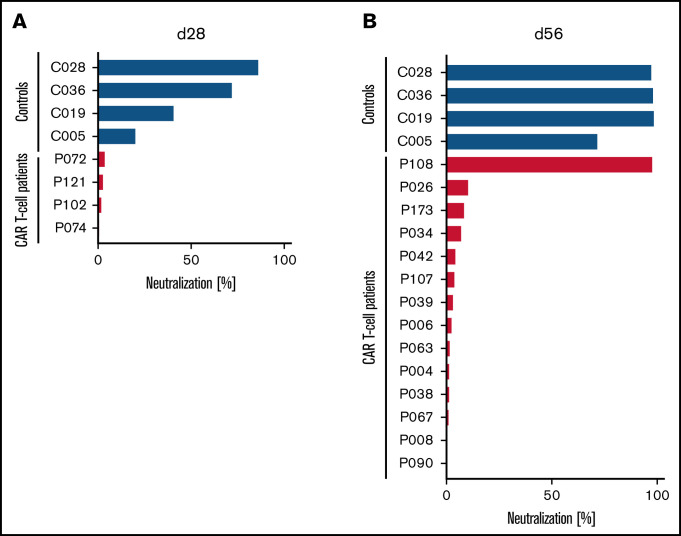
Figure 1.
Lack of SARS-CoV-2–neutralizing activity in patients treated with CD19-directed CAR T-cell therapy receiving mRNA vaccines. Neutralizing activity of vaccine-induced anti-RBD antibodies in the peripheral blood of patients treated with CD19-directed CAR T-cell therapy (red bars) and HCs (blue bars) after the first (A) or second (B) dose of the vaccine was measured as the degree of inhibition of interactions between RBD and angiotensin-converting enzyme-2 (ACE2). Additionally, for patients P072, P121, P102, and P074, day-84 samples showed complete lack of neutralizing antibodies. Of note, P108 (only patient with positive neutralization assay) had no detectable circulating B cells, suggesting the possibility of antibody production from nodal B cells or plasma cells. Neutralizing activity was measured using the cPass Neutralization Antibody Detection Kit (GenScript Biotech), which is a surrogate test detecting circulating neutralizing antibodies against SARS-CoV-2 that block the interaction between the RBD of the viral spike glycoprotein and the ACE2 cell-surface receptor.