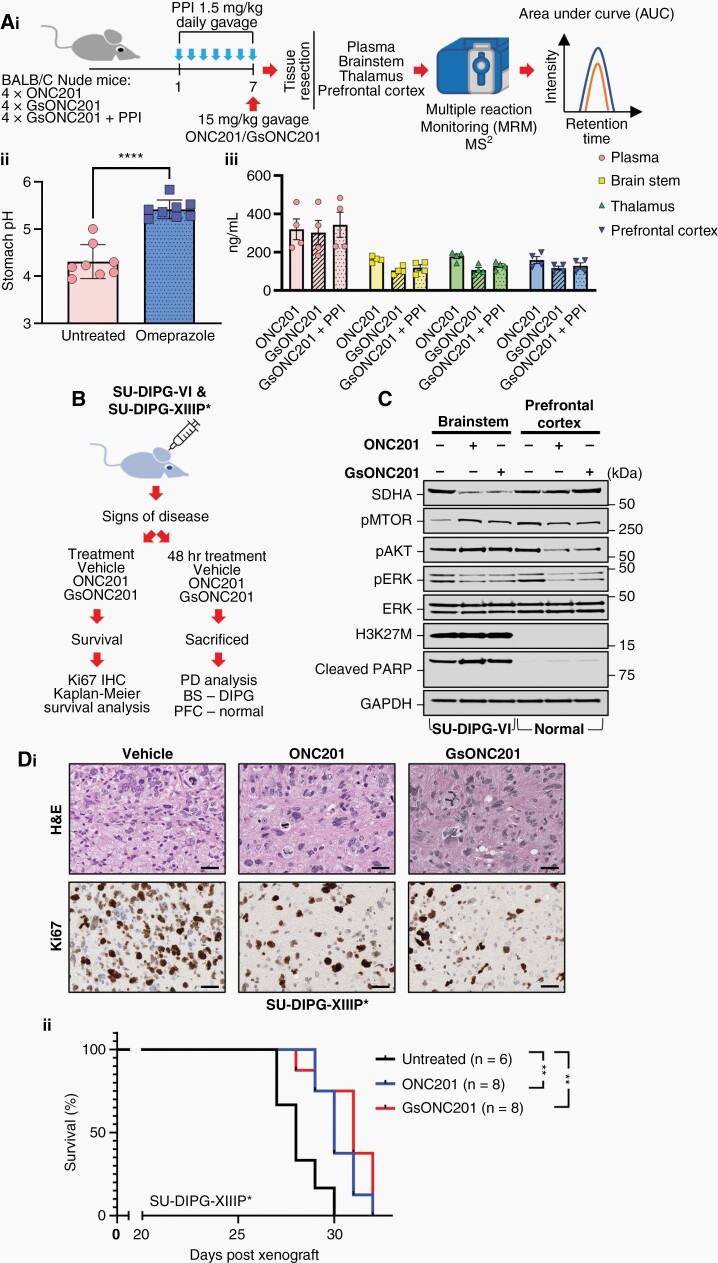
Figure 3.
Brain and tumor penetration of ONC201 and German-sourced ONC201 (GsONC201). (Ai) Schematic workflow for pharmacokinetic analysis of ONC201 and GsONC201 ± omeprazole (PPI). (Aii) pH of stomach contents of mice receiving oral 1.5 mg/kg omeprazole daily for 7 days. (Aiii) Multiple reaction monitoring (MRM) analysis of tissue concentrations of Oncoceutics or GsONC201 treated for 1 h prior to sacrifice 15 mg/kg, ± 7 days of prophylaxis administration of omeprazole. Average tissue concentrations; plasma 320.2 ng/mL, brainstem 140.2 ng/mL, thalamus 138 ng/mL, or prefrontal cortex 132 ng/mL. (B) Schematic workflow for pharmacodynamic, histological, and survival analysis of ONC201 and GsONC201. (C) Pharmacodynamic analysis of brainstem H3K27M+ SU-DIPG-VI and control prefrontal cortex tissues following oral treatment with vehicle, ONC201 and GsON201 125 mg/kg and sacrificed 48 h after treatment. (Di) Hematoxylin and eosin staining (H&E) and Ki67 staining of brainstem SU-DIPG-XIIIP* tumor tissue following 2 weeks of treatment with vehicle, ONC201 or GsON201 125 mg/kg. (Dii) Kaplan–Meier survival analysis of SU-DIPG-XIIIP* treated with vehicle, ONC201 or GsON201 125 mg/kg. Median survival of cohorts (days): Vehicle = 28, ONC201 = 31, GsONC201 = 32 (Log-rank [Mantel–Cox] test p values vs vehicle P = .0065 [95% CI 0.08419–1.2] and P = .0029 [95% CI 0.06278–1.042], respectively).