Abstract
Background
Peripheral arterial disease (PAD) is a manifestation of systemic atherosclerosis. Intermittent claudication is a symptomatic form of PAD that is characterized by pain in the lower limbs caused by chronic occlusive arterial disease. This pain develops in a limb during exercise and is relieved with rest. Propionyl‐L‐carnitine (PLC) is a drug that may alleviate the symptoms of PAD through a metabolic pathway, thereby improving exercise performance.
Objectives
The objective of this review is to determine whether propionyl‐L‐carnitine is efficacious compared with placebo, other drugs, or other interventions used for treatment of intermittent claudication (e.g. exercise, endovascular intervention, surgery) in increasing pain‐free and maximum walking distance for people with stable intermittent claudication, Fontaine stage II.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL databases and the World Health Organization International Clinical Trials Registry Platform and the ClinicalTrials.gov trials register to July 7, 2021. We undertook reference checking and contact with study authors and pharmaceutical companies to identify additional unpublished and ongoing studies.
Selection criteria
Double‐blind randomized controlled trials (RCTs) in people with intermittent claudication (Fontaine stage II) receiving PLC compared with placebo or another intervention. Outcomes included pain‐free walking performance (initial claudication distance ‐ ICD) and maximal walking performance (absolute claudication distance ‐ ACD), analyzed by standardized treadmill exercise test, as well as ankle brachial index (ABI), quality of life, progression of disease, and adverse events.
Data collection and analysis
Two review authors independently selected trials, extracted data, and evaluated trials for risk of bias. We contacted study authors for additional information.
We resolved any disagreements by consensus. We performed fixed‐effect model meta‐analyses with mean differences (MDs) and 95% confidence intervals (CIs). We graded the certainty of evidence according to GRADE.
Main results
We included 12 studies in this review with a total number of 1423 randomized participants. A majority of the included studies assessed PLC versus placebo (11 studies, 1395 participants), and one study assessed PLC versus L‐carnitine (1 study, 26 participants). We identified no RCTs that assessed PLC versus any other medication, exercise, endovascular intervention, or surgery. Participants received PLC 1 grams to 2 grams orally (9 studies) or intravenously (3 studies) per day or placebo.
For the comparison PLC versus placebo, there was a high level of both clinical and statistical heterogeneity due to study size, participants coming from different countries and centres, the combination of participants with and without diabetes, and use of different treadmill protocols. We found a high proportion of drug company‐backed studies. The overall certainty of the evidence was moderate.
For PLC compared with placebo, improvement in maximal walking performance (ACD) was greater for PLC than for placebo, with a mean difference in absolute improvement of 50.86 meters (95% CI 50.34 to 51.38; 9 studies, 1121 participants), or a 26% relative improvement (95% CI 23% to 28%). Improvement in pain‐free walking distance (ICD) was also greater for PLC than for placebo, with a mean difference in absolute improvement of 32.98 meters (95% CI 32.60 to 33.37; 9 studies, 1151 participants), or a 31% relative improvement (95% CI 28% to 34%). Improvement in ABI was greater for PLC than for placebo, with a mean difference in improvement of 0.09 (95% CI 0.08 to 0.09; 4 studies, 369 participants). Quality of life improvement was greater with PLC (MD 0.06, 95% CI 0.05 to 0.07; 1 study, 126 participants). Progression of disease and adverse events including nausea, gastric intolerance, and flu‐like symptoms did not differ greatly between PLC and placebo.
For the comparison of PLC with L‐carnitine, the certainty of evidence was low because this included a single, very small, cross‐over study. Mean improvement in ACD was slightly greater for PLC compared to L‐carnitine, with a mean difference in absolute improvement of 20.00 meters (95% CI 0.47 to 39.53; 1 study, 14 participants) or a 16% relative improvement (95% CI 0.4% to 31.6%). We found no evidence of a clear difference in the ICD (absolute improvement 4.00 meters, 95% CI ‐9.86 to 17.86; 1 study, 14 participants); or a 3% relative improvement (95% CI ‐7.4% to 13.4%). None of the other outcomes of this review were reported in this study.
Authors' conclusions
When PLC was compared with placebo, improvement in walking distance was mild to moderate and safety profiles were similar, with moderate overall certainty of evidence. Although In clinical practice, PLC might be considered as an alternative or an adjuvant to standard treatment when such therapies are found to be contraindicated or ineffective, we found no RCT evidence comparing PLC with standard treatment to directly support such use.
Plain language summary
Propionyl‐L‐carnitine for intermittent claudication
Background
Peripheral arterial disease (PAD), most often due to systemic atherosclerosis, affects 4% to 12% of the population aged 55 to 70 years and up to 20% of people over 70 years of age. Peripheral arterial disease tends to be more common in men overall, but woman have more asymptomatic PAD. Approximately 10% to 35% of those affected with PAD report intermittent claudication. Intermittent claudication is characterized by pain in the legs or buttocks that occurs with exercise and subsides with rest. Compared with age‐matched controls, people with intermittent claudication have a six‐fold increased risk of cardiovascular mortality. Treatment should include all measures of prevention for cardiovascular disease, which include cessation of smoking, exercise, and treatment for hypertension, diabetes, and cholesterol. Antiplatelet medications and statins are given to reduce the risk of cerebrovascular and coronary events.
To improve symptoms of claudication, regular (supervised) exercise and smoking cessation are the mainstay in the management of intermittent claudication. Drug treatments can include, besides antiplatelets and lipid‐lowering agents, vasoactive agents to improve blood flow, reduce pain, and improve walking distance. A minority of people with intermittent claudication undergo endovascular intervention or vascular surgery. Many pharmacological agents have been advocated for treating intermittent claudication, but none have gained worldwide acceptance. Few show some mild to moderate improvement in walking performance and are prudently proposed in the guidelines. Propionyl‐L‐carnitine (PLC) is a drug that may alleviate symptoms of PAD through a metabolic pathway, thereby improving exercise performance.
Key results
A search for relevant articles on propionyl‐L‐carnitine for treatment of intermittent claudication identified 12 relevant trials that matched our inclusion criteria (current until July 2021). In 11 studies, participants received either 1 gram to 2 grams oral PLC (9 studies) or intravenous propionyl‐L‐carnitine (3 studies) per day or placebo. One study compared propionyl‐L‐carnitine with L‐carnitine. Studies comparing PLC against other interventions such as exercise, other medication, endovascular intervention, or vascular surgery were not identified.
Maximum walking distance (or absolute claudication distance (ACD)) is the distance walked during a standardized test at which the participant stops walking due to muscular cramps. Pain‐free walking distance (or initial claudication distance (ICD)) is the distance walked during a standardized test until the start of pain. ACD and ICD were the outcomes of the review parameters and showed moderate improvement: for ACD, participants on propionyl‐L‐carnitine walked 50.86 meters or 26% farther than participants on placebo; for ICD, participants on propionyl‐L‐carnitine walked 32.98 meters or 31% farther than participants on placebo. Propionyl‐L‐carnitine participants showed improvement in ankle brachial index of 0.09 over placebo participants. Improvement in quality of life was also greater in the propionyl‐L‐carnitine group; however, this was based on the findings of only one study. Adverse events of propionyl‐L‐carnitine were similar to those of placebo and mainly consisted of nausea, gastric intolerance, and flu‐like symptoms. Propionyl‐L‐carnitine seemed to be a well‐tolerated and safe drug.
In the single propionyl‐L‐carnitine versus L‐carnitine study, participants on propionyl‐L‐carnitine showed significantly greater improvement in walking performance compared to those receiving L‐carnitine (ACD and ICD). This study did not report on the other outcomes of this review.
Certainty of the evidence
Overall certainty of the evidence was moderate (for propionyl‐L‐carnitine compared with placebo) or low (for propionyl‐L‐carnitine compared with L‐carnitine) because of differences between studies such as participants coming from different countries and centres, participants with and without diabetes, use of different treadmill protocols, small numbers of participants, and short follow‐up times, respectively.
Conclusion
When propionyl‐L‐carnitine was compared with placebo, improvement in walking distance was mild to moderate and safety profiles were similar, with overall moderate certainty of the evidence. Although in clinical practice, propionyl‐L‐carnitine might be considered a useful alternative medicine or addition to standard treatment when such therapies are contraindicated or ineffective, we found no clinical trial evidence comparing propionyl‐L‐carnitine with standard treatment to directly support such use.
Summary of findings
Background
Abbreviation list: see Appendix 1.
Description of the condition
Atherosclerosis is a common form of sclerosis in arteries, including those in the legs. Atheromas containing cholesterol, lipoid material, and lipophages are formed within the intima and the inner media of large and medium‐sized arteries, causing arterial narrowing and reducing blood flow to the lower limbs, at rest or during exercise. Peripheral arterial disease (PAD) is defined as occlusive atherosclerosis of the lower extremity arteries or the arteries distal to the aortic bifurcation (Hiatt 2008).
A widely accepted international classification of PAD is Fontaine’s classification (Fontaine 1954); asymptomatic patients are stage I, those with intermittent claudication (IC) are stage II, with rest pain stage III, and with trophic lesions stage IV. It is estimated that PAD occurs in approximately 12% of the adult population, and the prevalence increases with advancing age, such that almost 20% of people over 70 years of age have the disease (Hiatt 1995). In the United Kingdom, one in five of the late middle‐aged (65 to 75 years) population has evidence of PAD on clinical examination, although only a quarter of them have symptoms (Fowkes 1991). In a Swedish population‐based point‐prevalence study, women are reported to have higher prevalence of asymptomatic PAD and severe limb ischemia, but for intermittent claudication, there are no sex differences (Sigvant 2007).
The evolution from asymptomatic to symptomatic disease, the severity of symptoms in IC, and the transition to stages III and IV are influenced by the extent of anatomical narrowing of affected vessel(s) and the existence of a collateral circulation.
Intermittent claudication, stage II of Fontaine’s classification, is defined by leg muscle pain, cramping, and fatigue that are evoked by exercise and are relieved on rest. The overall incidence rate of IC is 6.4 per 1000 person‐years (Meijer 2002). The prevalence of IC appears to increase from about 3% in patients aged 40 years to 6% in patients aged 60 years (Norgren 2007). Intermittent claudication is a cardinal symptom of lower extremity PAD. It is caused by atherosclerosis in the peripheral arteries of the legs, leading to an insufficient blood supply during exercise, which causes anaerobic metabolism in the muscles with production of lactic acid and other metabolites. When PAD becomes more severe, the worsening ischemia leads to rest pain, ulceration, gangrene, and tissue loss. Intermittent claudication is indicative of systemic atherosclerosis, representing an independent risk factor for cardiovascular morbidity and mortality. The annual overall major cardiovascular event rate (myocardial infarction, ischemic stroke, and vascular death) in patients with IC is approximately 5% to 7% (Norgren 2007). One to three per cent of patients with IC will need major amputation within five years (Norgren 2007).
Strategies for management of symptomatic as well as asymptomatic PAD consist of conservative treatment with risk factor modification and exercise. For symptomatic PAD, treatment includes maximal risk factor modification, exercise, and, when indicated, invasive treatment consisting of balloon angioplasty with or without stenting and bypass surgery. Additionally, for symptomatic treatment, several pharmacological agents have been tested to improve walking capacity. The pharmacological management of IC is yet to be precisely defined. To date, drugs with proven efficacy for prevention of major cardiovascular and cerebrovascular events include antiplatelets, angiotensin‐converting enzyme (ACE) inhibitors, and lipid‐lowering drugs (mainly statins, which also have proven benefit for walking distance). Several oral vasoactive drugs claim to increase walking capacity among patients with IC, but robust data are lacking (Moher 2000). No single drug has gained full acceptance for its use in IC. Only oral naftidrofuryl and cilostazol have evidence (documented by Cochrane analyses) of moderately increased walking capacity (Brown 2021; De Backer 2010; De Backer 2012).
Description of the intervention
Vasoactive drugs may have a place in the pharmacological management of symptomatic PAD, in addition to lifestyle modification and basic cardiovascular pharmacotherapy, when revascularization is not indicated, when exercise therapy is not feasible, or when benefit is insufficient despite maximal treatment. In the current review, propionyl‐L‐carnitine is tested as a pharmacological agent for use in PAD.
Treatment should achieve improvement in functional capacity, that is, an increase in walking distance, reduced symptoms, enhanced quality of life, inhibition of the progression of atherosclerotic lesions, and reduction in cardiovascular and cerebrovascular morbidity and mortality. In keeping with European and American regulatory guidelines, improvement in functional capacity is assessed by measuring improvement in walking distance among patients.
How the intervention might work
Propionyl‐L‐carnitine is an acyl derivative, the propionyl ester of levo‐carnitine (L‐carnitine). Levo‐carnitine is an endogenous quaternary amine that is synthesized in the liver and kidneys.
Propionyl‐L‐carnitine has been postulated to improve walking capacity in patients with PAD by causing:
an increase in total carnitine content in ischemic muscle, improvement in muscle metabolism and stimulation of oxidative phosphorylation, and a decrease in plasma lactate concentration on exercise;
improvement in endothelial function; and
improvement in the micro‐architecture of the micro‐vascularization.
Under normal metabolic conditions, fuel substrates such as fatty acids and carbohydrates are converted to acyl–coenzyme A (CoA) intermediates to be used in Krebs’ cycle for complete oxidation. These CoA‐coupled intermediates are linked to the cellular carnitine pool through the reversible transfer of acyl groups between carnitine and CoA. One function of carnitine is to serve as a buffer to the acyl‐CoA pool by the formation of acylcarnitines (Hiatt 2004). Hence, during conditions of metabolic stress, incomplete oxidation or incomplete utilization of acyl‐CoA will lead to its accumulation (Hiatt 2004). L‐carnitine plays a crucial role in transporting fatty acids, which are coupled with acyl‐CoA, from the cytosol to the mitochondrial matrix for oxidative metabolism (Evans 2003).
Propionyl‐L‐carnitine has several actions.
It increases total carnitine content in ischemic muscle, improves muscle metabolism and stimulates oxidative phosphorylation, and decreases plasma lactate concentration on exercise.
-
Pharmacodynamic studies in patients with PAD show that propionyl‐L‐carnitine facilitates fatty acid oxidation by increasing intracellular levels of L‐carnitine, adenosine, and adenosine‐5'‐triphosphate (ATP) (Brevetti 1997). Maintaining the rate of fatty acid oxidation would permit glucose utilization to decrease, thus preserving muscle glycogen content and ensuring maximal rates of oxidative ATP production. Depletion of muscle glycogen has been linked to fatigue, thus glycogen preservation might be inherently performance enhancing (carnitine study; Brass 1998).
Within mitochondria, free carnitine, acting as an acetyl group buffer, reduces the acetyl‐CoA/CoA ratio with the formation of acylcarnitine, thus stimulating pyruvate dehydrogenase activity, because acetyl‐CoA is an end‐product inhibitor of pyruvate dehydrogenase. This acyl scavenging process, which requires adequate availability of carnitine, becomes crucial under conditions of limited oxygen availability when deficiency of free CoA limits the mitochondrial oxidation of both pyruvate and α‐ketoglutarate (and thus continuation of the pyruvate dehydrogenase complex and Krebs' cycle). The concurrent accumulation of CoA esters results in inhibition of the enzymes involved (Brass 1998). Accumulation of acetylcarnitine itself provides a store of acetyl groups, which are readily available for transacetylation back to acetyl‐CoA for utilization by Krebs’ cycle. Increased levels of short‐chain acylcarnitines, most often acetylcarnitine, occur in muscle and plasma of normal individuals performing maximal exercise. In patients with PAD stage II of Fontaine's classification, increased carnitine esterification with accumulation of acylcarnitines may occur even at rest (Brevetti 1996). The more severe the ischemic disease, the higher the accumulation of CoA esters in affected tissues, and consequently, the greater the amount of carnitine required for their removal. For such patients, carnitine supplementation restores normal carnitine homeostasis, improves the efficiency of oxidative phosphorylation, and lessens symptoms of claudication with concomitant improvement in walking capacity (Brevetti 1996; Brevetti 1999; Stephens 2007). Several studies have shown that administration of propionyl‐CoA increases glycogen (by blocking its utilization or promoting glycogen synthesis). Propionyl‐CoA is a gluconeogenesis substrate; thus it can generate glucose‐6‐phosphate, which can become glycogen. On the other hand, propionyl‐CoA can, by entering into Krebs’ cycle as succinyl‐CoA, provide additional substrates for energy metabolism with formation of ATP, thus leading to a glycogen‐sparing effect (Brevetti 1997).
Propionyl‐L‐carnitine improves endothelial cell function by reducing oxidative stress and reducing leucocyte activation and endothelial adhesion molecule expression (Milio 2009), and by providing protection against damage induced by ischemia and reperfusion, leading to maintenance of its regulatory role in vascular dynamics (Andreozzi 2009; Brass 1998).
Propionyl‐L‐carnitine improves the micro‐architecture of the micro‐vascularization, leading to improvement in the quality of micro‐vascular activity.
In studies included in the current review, PLC is administered as a tablet or by infusion.
Why it is important to do this review
A review assessing the evidence for efficacy of propionyl‐L‐carnitine in treatment of IC is warranted to determine whether the mechanisms described above translate into clinical benefit beyond the placebo effect. The bioavailability of L‐carnitine is uncertain. Therapy with L‐carnitine is not yet standardized, and results of existing studies are variable. In addition, propionyl‐L‐carnitine is an old drug, and its effectiveness remains unproven. Therefore, the question as to whether it is worthwhile to continue to use and further promote propionyl‐L‐carnitine for this indication should be raised.
Objectives
The objective of this review is to determine whether propionyl‐L‐carnitine is efficacious compared with placebo, other drugs, or other interventions used for treatment of intermittent claudication (e.g. exercise, endovascular intervention, surgery) in increasing pain‐free and maximum walking distance for people with stable intermittent claudication, Fontaine stage II.
Methods
Criteria for considering studies for this review
Types of studies
Double‐blind randomized controlled trials (RCTs) evaluating the efficacy of propionyl‐L‐carnitine in improving walking capacity among people with IC compared with placebo or versus other pharmacological or non‐pharmacological interventions were considered. Studies that failed on the risk of bias assessment, that is, studies that were judged to be at high risk of bias in one or more domains, were excluded from the review. See Assessment of risk of bias in included studies for more information on risk of bias.
Types of participants
People of either sex and of any age in whom IC (Fontaine stage II) due to atherosclerotic disease has been diagnosed by an expert clinician on clinical or investigative assessment (ankle brachial pressure index (ABI), exercise testing, duplex scanning, or angiography) (Fontaine 1954). Studies of people with asymptomatic lower limb atherosclerosis identified by testing were excluded. People with symptoms of critical limb ischemia (rest pain, skin ulcers, or gangrene) or who have undergone previous surgical intervention or percutaneous catheter interventions were not included.
Types of interventions
All types of propionyl‐L‐carnitine regimens versus placebo or versus some other pharmacological or non‐pharmacological intervention were included. Non‐pharmacological interventions might consist of exercise (including pneumatic compression), endovascular intervention, or surgery.
Types of outcome measures
The effect of propionyl‐L‐carnitine on walking capacity. Walking capacity can be assessed by two parameters: maximal walking distance (MWD) and pain‐free walking distance (PFWD). The primary outcome was MWD, and PFWD was a secondary outcome measure.
Primary outcomes
MWD, or absolute claudication distance (ACD) ‐ the distance walked during a standardized test at which the participant stops walking due to muscular cramps (maximum distance walked). Another parameter used for this is peak walking time (PWT)
Secondary outcomes
PFWD, or initial claudication distance (ICD) ‐ the distance walked during a standardized test (usually on a treadmill) until the onset of pain
Quality of life (QoL)
Progression of disease (to Fontaine stage III or IV or necessity for intervention (endovascular or surgical))
Side effects of propionyl‐L‐carnitine regimen
We assessed side effects using the same methods and eligibility criteria as were used for beneficial effects as described in Chapter 14.2.1 of the Cochrane Handbook for Systematic Reviews for Interventions (Higgins 2011). In addition, we checked the Primary Safety Update Reports (PSURs) via the European Medicines Agency (EMA) and the database of individual patient experience (DIPEx) registry for information on side effects.
Ankle brachial index, if available. ABI is considered a measure of the underlying hemodynamic severity of disease
Search methods for identification of studies
We applied no restriction on language, publication year, or publication status.
Electronic searches
The Cochrane Vascular Information Specialist first searched the following databases for relevant trials on March 22, 2017.
Cochrane Vascular Specialised Register.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5), in the Cochrane Library (www.cochranelibrary.com).
See Appendix 2 for details of the search strategy used to search CENTRAL.
The Information Specialist also searched the following trial registries for details of ongoing and unpublished studies using the search term 'propionyl' on March 22, 2017.
World Health Organization International Clinical Trials Registry who.int/trialsearch/.
ClinicalTrials.gov (clinicaltrials.gov/).
Current Controlled Trials (controlled-trials.com/).
The Cochrane Vascular Information Specialist subsequently conducted systematic top‐up searches of the following databases.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched from March 23, 2017, to July 7, 2021).
Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Register of Studies Online (CRSO; 2021, Issue 7).
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations; Ovid MEDLINE® Daily; and Ovid MEDLINE®) (searched from January 1, 2017, to July 12, 2021).
Embase Ovid (searched from January 1, 2017, to July 12, 2021).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Ebsco (searched from January 1, 2017, to July 12, 2021).
Allied and Complementary Medicine Database (AMED) Ovid (searched from January 1, 2017, to July 12, 2021).
The Information Specialist modelled search strategies for the listed databases on the search strategy designed for CENTRAL. When appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 6; Lefebvre 2011). Search strategies for major databases are provided in Appendix 3.
The Information Specialist also performed top‐up searches of the following trials registries on July 12, 2021.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch).
ClinicalTrials.gov (clinicaltrials.gov/).
Searching other resources
We reviewed all reference lists of identified studies and handsearched relevant bibliographies. In addition, we contacted study authors, trialists, and pharmaceutical companies marketing propionyl‐L‐carnitine for details on unpublished and ongoing trials and unpublished data. Given the fact that it is impossible to contact more than 50 suppliers worldwide, we restricted our first contact to one well‐known company in Italy (Pomezia) ‐ Sigma Tau ‐ which we contacted to request potential additional data.
In addition, we checked the PSURs via the EMA and the DIPEx registry for information on side effects (ema.europa.eu/ema; healthtalkonline.org).
Data collection and analysis
Selection of studies
The above search strategy yielded a set of potentially relevant articles. Two review authors (TDB and VK) independently selected RCTs on propionyl‐L‐carnitine for IC for inclusion in the review. Publications were selected based on the abstracts of retrieved articles or, if necessary, the original publication. Differences were resolved by consensus.
Data extraction and management
TDB and VK independently collected information from each included trial using data collection forms designed by Cochrane Vascular. We collected information on trial design, participant characteristics, inclusion and exclusion criteria, interventions and controls used, treatment periods, methods of assessment, and results of MWD and PFWD. We also collected data on QoL, progression of disease, side effects of the propionyl‐L‐carnitine regimen, and ABI. When necessary, we sought information from the authors of primary studies.
Assessment of risk of bias in included studies
TDB and VK independently assessed the risk of bias of included studies according to guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The original reports of all selected trials were transformed into structured abstracts. We evaluated studies for methods of randomization, concealment of allocation, blinding, description of withdrawals and dropouts in each group (internal validity), incomplete outcome data, and selective reporting. We assessed the risk of bias of selected studies by using the checklist for quality assessment from Cochrane Vascular. When necessary, in case of differences, a third assessor (RVS) or a fourth assessor (LVB) was involved, and consensus was reached. Studies that failed on the risk of bias assessment, that is, studies that we judged to be at high risk of bias in one or more domains, were excluded from the review.
Measures of treatment effect
We measured the main treatment effect using the mean difference (MD) of the walking distances (expressed in meters) (outcome data = continuous data (numerical quantity)). For studies using peak walking time, results were converted to distance using the velocity of the constant‐load treadmill settings. However, some studies use peak walking time on a graded treadmill, which is not comparable to MWD on a constant‐load treadmill. To resolve this problem and to be able to combine the data from studies with different treadmill protocols, we based the data on the treatment effect of the ratio (or percent change) of benefit of propionyl‐L‐carnitine relative to the benefit of placebo or control (or other active). Treatment effects are reported with a 95% confidence interval (CI).
Unit of analysis issues
The unit of the analysis was the individual participant.
Placebo‐controlled studies included in the review were parallel‐group RCTs. The L‐carnitine controlled study was a cross‐over study, which we included after analysis.
Dealing with missing data
Types of missing data can be missing studies, missing outcomes, missing summary data, missing individual results, or missing study level characteristics. We considered the reasons why data were missing. We considered missing data as either 'missing at random' or 'not missing at random'.
When possible, we contacted the original investigators to ask for any missing data. We planned to impute missing data with replacement values as if they were observed. For example, for missing main endpoint data, we contacted study authors and asked for available data or for the worst case value attributed to participants who interrupted the trial early for a reason related to PAD (progression to stages III and IV, aggravation of the disease, hospitalization, or surgery). For all other randomized participants who stopped for a reason unrelated to PAD, we used the last observation carried forward (LOCF), similar to most PAD trials. However, for sensitivity analysis purposes, we planned to carry out an alternative analysis by using summary statistics (mean of intermediate, non‐missing post‐baseline values) when at least two intermediate observations were available.
If missing data could not be retrieved, we analyzed only available data when the data could be assumed to be missing at random. If the data were not missing at random, we considered imputing the missing data with replacement values, or imputing the missing data (with uncertainty), or using specific statistical models that allowed for missing data, after consulting with and in consensus with our statistician.
When dealing with missing data, we also addressed the impact of the missing data on results when preparing the Discussion section of the review.
Assessment of heterogeneity
We assessed clinical heterogeneity by clinical judgement. We judged study design, participants, interventions, comparators, and outcomes, and stated whether or not trial data should be combined. This was the first step in determining whether a meta‐analysis was possible.
We identified statistical heterogeneity by using the I² statistic. I² describes the percentage of variability in point estimates that is due to heterogeneity rather than to sampling error. To explore any issues concerning heterogeneity, we considered several statistical models (including random‐effects and fixed‐effect models), as well as sensitivity analyses (described below).
Assessment of reporting biases
We planned to assess possible publication bias through simple funnel plots; however this was not possible because of an insufficient number of studies (Higgins 2011 suggests to make funnel plots if more than 10 studies are included in the meta‐analysis). A funnel plot is a simple scatter plot of intervention effect estimates from individual studies against some measure of each study's size or precision. The name 'funnel plot' arises from the fact that precision of the estimated intervention effect increases as the size of the study increases (Higgins 2011). When visual presentation of the funnel plot suggests a possible publication bias, we planned to calculate the correlation between effect size and sample size (Egger 1997).
Data synthesis
We pooled data on MWD and on PFWD from each trial to arrive at an overall estimate of the effectiveness of pharmacological interventions. We calculated the percentage change in walking distance before and after the interventions. We then calculated the mean difference with variance of the propionyl‐L‐carnitine group compared with the control group. If feasible, we conducted a fixed‐effect model meta‐analysis with extracted results.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analysis, according to the participant characteristic diabetes versus non‐diabetes.
We planned to perform subgroup analyses for treatment duration, dose (for propionyl‐L‐carnitine, a wide dose range exists, ranging from 1 gram daily to 4 grams daily), and route of administration (orally or intravenously).
Sensitivity analysis
Studies at high risk of bias were excluded from the review.
Depending on study selection, we planned to also test the stability of results by comparing results while (1) using all available included trials, (2) excluding trials contributing large weight to the analyses, and (3) considering full intention‐to‐treat (ITT) analysis versus per‐protocol (PP) analysis in trials.
As described above, in the Dealing with missing data section, we planned to carry out an alternative analysis by using summary statistics (mean of intermediate, non‐missing post‐baseline values) when at least two intermediate observations were available.
As a post‐hoc measure, we performed an additional sensitivity analysis to explore more fully the high heterogeneity that was evident in many analyses. This sensitivity analysis included only the three studies contributing the largest weights to the fixed‐effect model.
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2008), which takes into account the following five criteria: risk of bias, inconsistency, imprecision, publication bias, and directness of results. For each comparison, we rated the certainty of evidence for each outcome as 'high', 'moderate', 'low', or 'very low' using GRADEpro GDT. We presented a summary of evidence for main outcomes in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2011). When meta‐analysis was not possible, we presented results in a narrative manner.
We included in the 'Summary of findings' table data for the following outcomes: absolute claudication distance, initial claudication distance, quality of life, progression of disease (to Fontaine stage III or IV or necessity for intervention (endovascular or surgery)), side effects of the propionyl‐L‐carnitine regimen, and ABI.
Results
Description of studies
Results of the search
See Figure 1.
1.
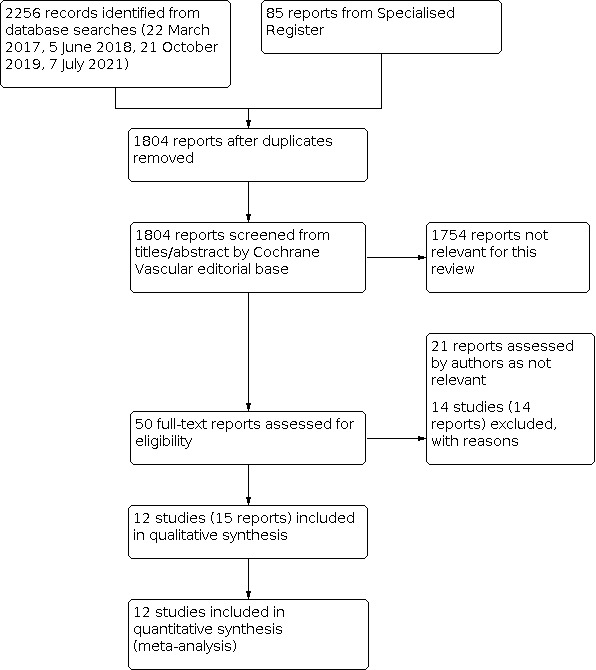
Study flow diagram.
In total, we assessed 50 full‐text papers for inclusion. We included 12 studies (15 reports) and excluded 14 studies (14 reports). We assessed the remaining 21 reports as not relevant.
Included studies
See Characteristics of included studies.
We included 12 studies (15 reports) that fulfilled all inclusion criteria (Andreozzi 2008; Brevetti 1992; Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011; Luo 2013; Signorelli 2006a; Signorelli 2006b). All studies were RCTs comparing propionyl‐L‐carnitine (PLC) with placebo or another treatment. In most cases, the comparator was placebo. In one trial, PLC was compared against L‐carnitine (Brevetti 1992). Studies comparing PLC against other interventions such as exercise, other medications, endovascular intervention, or surgery were not identified.
The 12 included studies randomized a total of 1423 participants: PLC versus placebo: 1395 participants; and PLC versus other: 28 participants. For the primary outcome ACD, a total of 1257 participants were included (ITT), dropping to 1121 participants after dropouts were taken into account (PP population, 10.8% dropouts). For the secondary outcome ICD, a total of 1287 participants were included (ITT), dropping to 1151 participants after dropouts were taken into account (10.6% dropouts). The number of participants in these studies ranged from 19 in Dal Lago 1999 and Greco 1992 to 282 in Luo 2013. Signorelli 2006a reported ICD and ABI as primary outcomes. Andreozzi 2008 reported only ACD as an outcome parameter. Most trials were performed in Italy (Andreozzi 2008; Brevetti 1992; Brevetti 1995; Coto 1992; Dal Lago 1999; Greco 1992; Signorelli 2006a; Signorelli 2006b); the other trials were performed in the United States ‐ Hiatt 2001; Hiatt 2011 ‐ and in China ‐ Luo 2013.
PLC treatment was similar across trials, most often 1 gram or 2 grams PLC daily. In three trials, PLC was given intravenously (Andreozzi 2008; Brevetti 1992; Signorelli 2006b), and in nine trials, PLC was taken orally (Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011; Luo 2013; Signorelli 2006a). Follow‐up in most studies was six months to one year. Evaluation of walking parameters was performed on a treadmill, but characteristics of testing varied because of different slopes and speeds of the treadmill.
Andreozzi 2008 ‐ This placebo‐controlled RCT included 44 people (22 intervention participants, 22 control participants) with IC. Half of the participants suffered from severe claudication (MWD < 100 m) and half suffered from moderate claudication (MWD < 200 m), resulting in four equal subgroups of 11 participants each, based on intervention, control, and severity of claudication. The intervention consisted of physical training three times weekly for six weeks + intravenous (iv) saline solution + iv 600 mg PLC three times weekly during the last three weeks of training. Control participants received physical training three times weekly for six weeks + iv saline solution three times weekly during the last three weeks of training. MWD on a treadmill (speed 2.5 km/h, slope 15%) was the outcome of this study. Measurements were performed at baseline, and after three and six weeks.
Brevetti 1992 ‐ This cross‐over study is a double‐blind RCT comparing PLC with L‐carnitine (LC). Twelve people with IC were enrolled in the preliminary dose‐finding study and 14 people with IC in the comparative study (iv 600 mg PLC versus iv 500 mg LC, which is an equimolar dose). Participants were referred to the outpatient clinic of the study authors. In the preliminary study, all participants first received iv placebo, followed, four days later, by intervention: iv 300 mg PLC or 600 mg PLC (cross‐over after four days between 300 mg PLC and 600 mg PLC arms, following a washout period). In the comparative study, all participants first received iv placebo, four days later followed by intervention: iv 600 mg PLC or iv 500 mg LC (cross‐over after four days between the two intervention arms) without a washout period. Outcomes were MWD (meters) and PFWD (meters) on the treadmill (2.5 mph, slope 7%) and a hemodynamic assessment (ABI, cross‐sectional area (CSA) of the common femoral artery, blood flow velocity (cm/s), blood flow rate (mL/min), pulsatility index (PI), and resistance index (RI)). In this study, it should be noted that study duration and follow‐up were remarkably short (four days) and the number of participants low. Funding was provided by the pharmaceutical company Sigma Tau.
Brevetti 1995 ‐ Randomized placebo‐controlled double‐blind trial with a dose‐titration design performed at 13 centers. 245 (ITT) (214 (PP)) people with IC underwent a two‐week washout phase in which they were familiarized with the treadmill. 31 dropouts from the study were reported (14 due to various adverse events, 17 due to poor compliance). Intervention participants (n = 99) received oral PLC 1/2/3 g/d for six months, and controls (n = 115) received oral placebo 1/2/3 g/d for six months. The initial dose of 2 × 500 mg daily was increased at two‐monthly intervals to 2 grams daily, then to 3 grams daily, for participants with improvement in treadmill performance less than 30% over baseline; participants showing improvement of 30% or greater continued with the same dose as in the previous two months. Study outcomes were MWD and PFWD on the treadmill (speed 4 km/h, slope 7%) and analysis of the titration course: probability of obtaining an increase in MWD of 30% or greater with a specific dose. Funding by Sigma Tau. Quality of life was measured using the McMaster Health Index Questionnaire at the end of the run‐in period and at the last control visit. This is a self‐administered generic questionnaire of 59 health‐related statements covering physical, social, and emotional dimensions. QoL results were reported by Brevetti 1997.
Brevetti 1999 ‐ This double‐blind, randomized, placebo‐controlled trial included 485 (ITT) (328 (PP)) participants with IC for a minimum of one year. 157 dropouts were observed: 10 participants died, 57 dropped out due to adverse events, 61 dropped out because of protocol violation, and 29 participants were lost to follow‐up. Dropout ratios were balanced between the two treatment arms. Participants were stratified on the basis of MWD at baseline (cutoff point 250 m) and MWD variability at baseline (cutoff point 25%) into four groups (S1 to S4). 485 participants were considered in the ITT protocol, and 328 completed the one‐year protocol. Participants taking less than 75% of prescribed dosis were considered as dropouts. Intervention participants (n = 162) received oral PLC 1 gram twice daily for one year; the control group (n = 166) received placebo oral 1 gram twice daily for one year. Study outcomes were MWD and PFWD on the treadmill (slope 7%, speed 3 km/h), QoL, and adverse events. Measurements were performed every two months. Funding was provided by Sigma Tau. For results of walking distance, only the S1 group (MWD < 250 m, variability < 25%) was considered; for adverse events reporting, all four groups were included.
Coto 1992 ‐ This double‐blind, randomized, placebo‐controlled trial was conducted with participants from seven centers: 300 (ITT) (282 (PP)) participants with IC for a minimum of one year, randomized in two groups. 18 dropouts were reported (due to adverse events or poor collaboration). Intervention participants (n = 140) received oral PLC 2 × 1 g/d for six months; control participants (n = 142) received oral placebo 2 × 1 g/d for six months. Primary outcomes were MWD and PFWD on the treadmill (speed 3 km/h, slope 7%).
Dal Lago 1999 ‐ A double‐blind, randomized, placebo‐controlled trial with 22 (ITT) (19 (PP)) participants with IC for a minimum of one year, baseline MWD between 150 and 400 meters, and ABI less than 0.80. The intervention arm received oral PLC 1 g/d for 90 days; the control arm received oral placebo 1 g/d for 90 days. MWD and PFWD on the treadmill (speed 4 km/h, slope 4%) were the study outcomes.
Greco 1992 ‐ This RCT was performed double‐blind on 20 diabetic individuals with IC for a minimum of one year, ABI less than 0.75, MWD from 100 to 500 meters, and less than 20% variation in ACD during the washout period. 20 participants were randomized into two groups of 10 participants. One dropout from the placebo group was reported. The intervention (n = 10) consisted of oral PLC 1.5 g/d for six months; the control (n = 10) consisted of oral placebo 1.5 g/d for six months. Main outcomes were MWD and PFWD measured on the treadmill (slope 10%, speed 2.5 km/h). Funding was provided by Sigma Tau.
Hiatt 2001 ‐ This double‐blind, randomized, placebo‐controlled trial contains 161 (ITT) (155 (PP)) participants with IC recruited from 10 centers in the United States (six centers) and Russia (four centers). There were six dropouts due to losses to follow‐up. The intervention group (n = 82) received oral PLC 2 g/d for six months; the control group (n = 73) received oral placebo 2 g/d for six months. Peak walking time (PWT) and claudication onset time (COT) were recorded on the treadmill (speed 2 mph, slope 12%). Funding was provided by Sigma Tau.
Hiatt 2011 ‐ A double‐blind, randomized, placebo‐controlled multi‐center trial with 69 randomized participants between 40 and 80 years old with IC for a minimum of one year and PWT between 90 and 360 seconds at baseline (modified ITT analysis on 62 participants who underwent at least one post‐randomization treadmill test, seven dropouts due to adverse events and study withdrawal). The intervention (n = 32) consisted of oral PLC 2 g/d + instruction on home‐based physical exercise three times weekly, for a duration of six months. Control (n = 30) consisted of oral placebo 2 g/d + instruction on home‐based physical exercise three times weekly for six months. Study outcomes were PWT and COT on the treadmill (speed 2 mph, slope increase 2%/2 min). Home‐based physical exercise was checked with a Stepwatch activity monitor. Funding was provided by Sigma Tau.
Luo 2013 ‐ This is a double‐blind, randomized, multi‐center, phase 3, parallel‐group study with 239 (full analysis set: all participants with at least one post‐baseline assessment) (212 (PP set: all participants who completed the trial, dropouts due to adverse events in both groups)) participants with IC with a baseline MWD between 50 and 250 meters and baseline ABI less than 0.90. Intervention participants received oral PLC 2 g/d for four months (n = 103), control participants received oral placebo 2 g/d for four months (n = 109). ABI, PWT, and COT were the study outcomes, treadmill speed and slope were not given, and speed of 2 mph was assumed (as used in eligibility tests for MWD). Funding was provided by the Lee pharmaceutical company.
Signorelli 2006a ‐ This study is a double‐blind, randomized, placebo‐controlled clinical trial with 74 participants with non‐insulin‐dependent diabetes mellitus (NIDDM)‐associated PAD (stage 2 Leriche classification). Intervention (n = 37) consisted of oral PLC 2 g/d for one year; control (n = 37) consisted of oral placebo 2 g/d for one year. ABI and PFWD were evaluated on the treadmill (speed 3.5 km/h, slope 7.5%). These measurements were performed at baseline and at 6 and 12 months.
Signorelli 2006b ‐ This trial is a randomized, placebo‐controlled, double‐blind clinical trial of 64 participants with IC on hemodialysis (chronic kidney insufficiency). The intervention group (n = 32) received iv PLC 600 mg in saline solution three times/week for one year; the control group (n = 32) received placebo (only iv saline solution infusion) three times/week for one year. No walking distances were reported in this study; the only outcome of interest for this review was ABI.
Studies comparing PLC against other interventions such as exercise were not identified.
Excluded studies
See Characteristics of excluded studies.
In total, we excluded 14 studies for these reasons (Allegra 2008; Barker 2001; Brevetti 1984; Brevetti 1988; Brevetti 1989; Brevetti 1996; Goldenberg 2006; JPRN‐UMIN000016267; Loffredo 2006; Loffredo 2013; Ragozzino 2004; Riccioni 2008; Strano 2002; Taylor 1996).
Study was not an RCT: Allegra 2008, JPRN‐UMIN000016267, Ragozzino 2004, and Taylor 1996.
No double‐blinding was noted: studies were not double‐blind (Riccioni 2008 and Strano 2002), or it is unclear if they were double‐blind (Barker 2001).
Intervention drug in the study (L‐carnitine) was not the intervention in this review (propionyl‐L‐carnitine): Brevetti 1984, Brevetti 1988, Brevetti 1989, and Goldenberg 2006.
Outcome parameter was not a primary or secondary outcome as set for this review: Brevetti 1996 (metabolic blood markers), Loffredo 2013 (flow‐mediated dilation).
Study failed on the risk of bias assessment: Barker 2001, Loffredo 2006, Riccioni 2008, and Strano 2002. Also, Barker 2001, Riccioni 2008, and Strano 2002 have either no blinding or unclear blinding of investigators as listed above.
Reasons for failing the risk of bias assessment include the following.
Barker 2001: incomplete outcome data (high risk: no P values for walking results), selective reporting (high risk: no further reporting on ABI; walking distances reported as coefficients of variation, which was not prespecified), blinding (unclear risk: unclear whether there was blinding on the investigators' side), other bias (high risk: very small population (six participants)).
Loffredo 2006: incomplete data (high risk: no P values comparing PLC and placebo), selective reporting (unclear risk: focus of this study on oxidative stress, less on walking capacity), other bias (unclear risk: very low number of participants (10), short follow‐up/treatment (7 days' treatment iv), cross‐over study: 3 days placebo ‐ 3 days PLC). Given the high risk of bias, the cross‐over design with short washout period, and the low number of participants, we hypothesize that a sensitivity analysis with this study might not be relevant and will not change the results.
Riccioni 2008: selection bias: no reporting of random sequence generation, no reporting of allocation concealment; blinding (high risk: no blinding), incomplete outcome data (high risk: no standard deviations); PLC monotherapy regimen alone or in association with pulsed muscular compression compared to physical therapy by itself: three arms: (1) infusional PLC therapy at a dosage of 4 fl (total: 1200 mg PLC) in 250 cc of physiological solution five days a week for four weeks; (2) treatment with PLC in association with pulsed muscular compression therapy by Vascupump (five sessions a week for four weeks); and (3) submission only to Vascupump.
Strano 2002: blinding (high risk: no blinding), incomplete outcome data (high risk: no absolute numbers given ‐ only mean differences, without standard deviations; figures do not match results for the mean difference; follow‐up ABI values not given), selective reporting (high risk: major difference between ITT and PP populations (114 versus 68), indicating that results might be valuable only for a select group of highly motivated participants).
Risk of bias in included studies
The risk of bias assessment was performed according to the Cochrane guidelines described by Higgins 2011. The individual assessment for each study can be found in the risk of bias table under Characteristics of included studies. Insufficient information was the main reason for rating "unclear risk." See Figure 2 and Figure 3 for a graphical presentation of the risk of bias.
2.

Risk of bias graph of the included studies: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In four trials, the randomization method was given in sufficient detail (low risk) (Dal Lago 1999; Hiatt 2001; Signorelli 2006a; Signorelli 2006b). The method used to generate allocation concealment was unclear in all trials (Andreozzi 2008; Brevetti 1992; Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011; Luo 2013; Signorelli 2006a; Signorelli 2006b).
None of the 12 included studies reported on allocation concealment.
Blinding
Eleven trials were at low risk for performance bias, as there was blinding of personnel and participants (Brevetti 1992; Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011; Luo 2013; Signorelli 2006a; Signorelli 2006b). For the same 11 studies, blinding of outcome assessment was assumed, indicating low risk for detection bias. For only one study, risk for performance and detection bias was unclear due to lack of information (Andreozzi 2008).
Incomplete outcome data
All 12 included studies were at low risk for attrition bias, as all results for all (sub)groups were given (Andreozzi 2008; Brevetti 1992; Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011; Luo 2013; Signorelli 2006a; Signorelli 2006b).
Selective reporting
Only five trials had low risk of reporting bias (Andreozzi 2008; Greco 1992; Luo 2013; Signorelli 2006a; Signorelli 2006b). For the other seven trials, there was a possibility of selective reporting, indicating unclear risk due to various reasons (see "Risk of bias table") (Brevetti 1992; Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Hiatt 2001; Hiatt 2011).
Other potential sources of bias
One trial reported pharmaceutical company funding and also explicitly reported the absence of conflict of interest (although there was company sponsoring) (Luo 2013). Eight trials do report on company funding and therefore might have a possible conflict of interest (unclear risk) (Andreozzi 2008; Brevetti 1992; Brevetti 1995; Brevetti 1999; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011). Three trials do not report any funding and do not provide a conflict of interest statement (Coto 1992; Signorelli 2006a; Signorelli 2006b).
The main analysis of Brevetti 1995 was not split into subgroups of severe and moderate claudication, as was done in an additional publication for the same population (Brevetti 1997). A pronounced outcome discrepancy was shown between groups A (98 participants coming from 1 center) and B (116 participants coming from 12 centers). The study authors themselves state that "a marked center effect was observed because the 77 patients studied in 1 center were more severely affected than those studied in the remaining 12 centers." A conflict of interest is possible because the study was supported by Sigma Tau.
Effects of interventions
Summary of findings 1. Propionyl‐L‐carnitine compared to placebo for intermittent claudication.
| Propionyl‐L‐carnitine compared to placebo for intermittent claudication | ||||||
| Patient or population: people with intermittent claudication Setting: outpatient setting Intervention: propionyl‐L‐carnitine Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with placebo/control | Risk with propionyl‐L‐carnitine | |||||
| Absolute claudication distance (MWD + PWT) (in meters) Follow‐up: 3 weeks to 1 year |
Mean change in ACD in placebo group was 59.89 m | MD 50.86 m higher (50.34 higher to 51.38 higher) | ‐ | 1121 (9 studies) | ⊕⊕⊕⊝ moderatea | |
| Initial claudication distance (PFWD + COT) (in meters) Follow‐up: 90 days to 1 year |
Mean change in ICD in placebo group was 32.24 m | MD 32.98 m higher (32.6 higher to 33.37 higher) | ‐ | 1151 (9 studies) | ⊕⊕⊕⊝ moderatea | |
| Quality of life Follow‐up: 6 months |
Mean change in QoL score in placebo group was 0.01 | MD 0.06 higher (0.05 higher to 0.07 higher) | ‐ | 126 (1 study) | ⊕⊕⊕⊝ moderateb | |
| Progression of disease (to Fontaine stage III or IV, or necessity for intervention (endovascular or surgery)) Follow‐up: 1 year |
1 study evaluated progression of disease: 5/242 (2%) PLC participants evolved from Fontaine stage II to stage III (rest pain) vs 10/243 (4%) placebo participants; 2/242 (0.8%) PLC participants evolved to Fontaine stage IV (critical ischemia) vs 0/243 (0%) placebo participants | 485 (1 study) |
⊕⊕⊝⊝ lowc |
|||
| Side effects of propionyl‐L‐carnitine regimen Follow‐up: 6 months to 1 year |
Brevetti 1995: 7 AEs in placebo group and 5 AEs in PLC group not requiring drug discontinuation. Nausea and gastric pain were the most frequent side effects; 11 AEs resulting in drug discontinuation occurred in the PLC group, and 3 in the placebo group. According to study authors, medical problems requiring drug discontinuation in the PLC group were unrelated to study medication Brevetti 1999: 27 PLC participants discontinued the study because of the occurrence of serious AEs (mainly cardiac and peripheral vascular). In the placebo group, 30 AEs required study discontinuation. 38 AEs not requiring drug discontinuation occurred in the PLC group vs 98 in the placebo group; flu syndrome was the most frequent AE without a difference in occurrence between arms Coto 1992: 3 AEs requiring study interruption in the PLC group and 6 in the placebo group. The most common AE was abdominal pain Hiatt 2001 and Hiatt 2011: no mention of important differences in side effects between 2 intervention arms: 70% of PLC participants and 68% of placebo participants experienced 1 or more AEs; AEs that affected more than 5% of participants in either group with a ratio > 1.5 PLC vs placebo included nausea, diarrhea, bronchitis, and back pain Signorelli 2006b: no AEs |
1303 (6 studies) |
⊕⊕⊕⊝ moderated |
Overall, PLC appears to be a safe and well‐tolerated drug, as no significant differences with placebo can be found in studies with follow‐up from 6 months to 1 year | ||
| Ankle brachial index (ABI) Follow‐up: 4 months to 1 year |
Mean ABI in control group was ‐0.02 | MD 0.09 higher (0.08 higher to 0.09 higher) | ‐ | 369 (4 studies) | ⊕⊕⊕⊝ moderatee |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ABI: ankle brachial index; ACD: absolute claudication distance; AE: adverse event; CI: confidence interval; COT: claudication onset time; ICD: initial claudication distance; MD: mean difference; MWD: maximum waking distance; PFWD: pain‐free walking distance; PLC: propionyl‐L‐carnitine; PWT: peak walking time; QoL: quality of life. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level due to high heterogeneity between studies. Furthermore, the overall estimate of effect reflects mainly results of the studies of Brevetti (largest numbers of participants); however in these studies, subgroups were not reported (?publication bias) and there was a marked center effect. Overall results were relatively consistent among studies. The same remarks apply for all outcomes. bDowngraded by one level because this was investigated in only one study. cDowngraded by two levels because this outcome was investigated in only one study and the absolute number of events in this study was low. dDowngraded by one level because causal relationship of different adverse events was not fully explained. eDowngraded by one level because this outcome was investigated in a small number of studies (four studies).
Summary of findings 2. Propionyl‐L‐carnitine compared to L‐carnitine for intermittent claudication.
| Propionyl‐L‐carnitine compared to L‐carnitine for intermittent claudication | ||||||
| Patient or population: people with intermittent claudication Setting: outpatient setting Intervention: propionyl‐L‐carnitine Comparison: L‐carnitine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with L‐carnitine | Risk with propionyl‐L‐carnitine | |||||
| Absolute claudication distance (in meters) Follow‐up: 4 days |
Mean ACD in control group was 36.00 m | MD 20 m higher (0.47 higher to 39.53 higher) | ‐ | 28 (1 study) | ⊕⊕⊝⊝ lowa |
|
| Initial claudication distance (in meters) Follow‐up: 4 days |
Mean ICD in control group was 23.00 m | MD 4 m higher (9.86 lower to 17.86 higher) | ‐ | 28 (1 study) | ⊕⊕⊝⊝ lowa |
|
| Quality of life | See comments | ‐ | ‐ | ‐ | The single included study in this comparison did not report this outcome | |
| Progression of disease (to Fontaine stage III or IV or necessity for intervention (endovascular or surgery)) | See comments | ‐ | ‐ | ‐ | The single included study in this comparison did not report this outcome | |
| Side effects of propionyl‐L‐carnitine regimen | See comments | ‐ | ‐ | ‐ | The single included study in this comparison did not report this outcome | |
| Ankle brachial index | See comments | ‐ | ‐ | ‐ | The single included study in this comparison did not report this outcome | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACD: absolute claudication distance; CI: confidence interval; ICD: initial claudication distance; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels because results are based on a single study with borderline or no statistically significant results. Moreover, this study is a cross‐over study with few participants and limited follow‐up time (four days), as well as several risk of bias issues.
PLC versus placebo or control
Absolute claudication distance
In this review, under the common term "absolute claudication distance" (ACD), we unite all results from different studies that evaluate maximal walking performance. This includes all maximal walking distance (MWD) (in unit of length) results as well as all peak walking time (PWT) (in unit of time) results from the included studies. All time measurements from PWT studies were converted to absolute distances (in meters), according to treadmill testing speed. This might be subject to discussion given the fact that for example studies that use PWT on a graded treadmill are not comparable to studies that use MWD on a constant‐load treadmill. However, different treadmill protocols exist within studies measuring MWD and within those measuring PWT. Therefore, we also based the data on the treatment effect of the ratio (or percent change) of the benefit of propionyl‐L‐carnitine (PLC) relative to the benefit of placebo or control (or other active) treatment.
Selection of relevant and reliable studies resulted in nine included studies reporting on ACD (Andreozzi 2008; Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011; Luo 2013). In total, this participant pool contained 1257 participants (627 PLC participants, 630 placebo control participants) who were randomized for supplementation (intention‐to‐treat (ITT) population). 1121 participants or 89.2% completed the trials and could be administered for further full analysis (per‐protocol (PP) population). In light of this participant pool, randomization in the studies created two balanced groups that were comparable in number, age, and sex. The following baseline clinical parameters matched well: baseline ACD 186 meters for the PLC group, 191 meters for the placebo group, and baseline ankle brachial index (ABI) mean equal to 0.63 in both intervention arms. The dropout ratio differed (12.1% versus 9.5%); however, this results mainly from inequalities in the Brevetti 1995 and Luo 2013 studies. Reasons for dropout in these studies are given; dropout is likely to be due to coincidence.
Detailed results can be found under Analysis 1.1. Analysis was performed on 1121 participants from nine different studies. As mentioned above, time measurements in PWT were converted to units of length. In the PLC group, participants walked 122.7 meters more at the end of the study compared to baseline, versus 59.7‐meter improvement in the control group versus baseline. Considering the relative weight of each study, this resulted in a mean difference of 50.86 meters (95% confidence interval (CI) ranging from 50.34 to 51.38) favoring PLC (moderate‐certainty evidence). (See Figure 4.) In this analysis, Brevetti 1995 is very prominent in determining the outcome of this comparison. Due to its small confidence intervals, it has a relative weight of 75%. This gives other large studies such as Coto 1992 and Hiatt 2001 less weight in the review. This trend is seen in all analyses.
1.1. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 1: Absolute claudication distance (MWD + PWT)
4.

Forest plot of comparison: 1 Propionyl‐L‐carnitine versus placebo or control, outcome: 1.1 Absolute claudication distance (MWD + PWT).
Due to slight differences in treadmill testing protocols (slope, speed), baseline walking performances are heterogeneous. To cope with these diverse results, not only absolute but also relative improvements in walking distance were calculated (in odds ratios). For each intervention arm, relative improvement in walking distance versus baseline was calculated. (See Analysis 1.2.) PLC participants showed a mean improvement of 1.61, and placebo control participants showed a mean improvement of 1.29; this means that PLC participants walked 1.61 times better (61% more distance) at the end of the studies than at baseline and placebo/control participants walked 1.29 times better (29% more distance) at the end of the study than at baseline. After the weight of each study is considered again, the mean difference results in a significant advantage of 0.26 for PLC (95% CI 0.23 to 0.28) or 26% more improvement compared with the control arm. Again Brevetti 1995 is very prominent in the outcome of this comparison (Figure 5).
1.2. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 2: ACD (odds ratio)
5.

Forest plot of comparison: 1 Propionyl‐L‐carnitine versus placebo or control, outcome: 1.2 ACD (odds ratio).
We note that heterogeneity/I² (percentage of total variation across studies that is due to heterogeneity rather than to chance) for all outcomes is very high (> 85% for all studies that have pooled data).
Initial claudication distance
Similar to the analysis process for ACD, under the common term "initial claudication distance" (ICD), we unite all results from the different studies that evaluate pain‐free walking performance. This includes all pain‐free walking distances (in unit of length) results, as well as all claudication onset time (in unit of time) results, from the included studies. All time measurements from COT studies were converted to absolute distances (in meters), according to treadmill testing speed.
For this analysis, again nine studies were included (Brevetti 1995; Brevetti 1999; Coto 1992; Dal Lago 1999; Greco 1992; Hiatt 2001; Hiatt 2011; Luo 2013; Signorelli 2006a). Compared to the ACD analysis, one study was removed ‐ Andreozzi 2008 ‐ and one study was added ‐ Signorelli 2006a. The participant pool contained 1287 ITT participants and was well balanced between intervention arms (642 in the PLC group, 645 in the placebo group). No major differences between the two groups could be found in demographic and clinical parameters. Mean baseline ICDs were 120.3 meters (PLC) versus 120.8 meters (placebo).
Detailed results can be found under Analysis 1.6 (Figure 6). Per‐protocol analysis was performed on 1151 participants, excluding 136 dropout participants. As mentioned above, time measurements in COT were converted to distances in units of length. The improvement in walking performance in absolute numbers for PLC participants was 75.7 meters; for control participants in the placebo arm, mean improvement was 36.6 meters. Weighing the impact of the studies revealed a mean difference of 32.98 meters (95% CI 32.60 to 33.37) favoring PLC (moderate‐certainty evidence). In keeping with the same methods used for ACD, relative changes were calculated (Analysis 1.7). This resulted in a relative improvement of 1.67 for PLC participants versus 1.32 for control participants. Mean difference between intervention arms is 0.31 (95% CI 0.28 to 0.34), or 31% more improvement in favor of PLC participants. These numbers favoring PLC over placebo confirm results from the ACD analysis. See also Figure 7.
1.6. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 6: Initial claudication distance (PFWD + COT)
6.

Forest plot of comparison: 1 Propionyl‐L‐carnitine versus placebo or control, outcome: 1.6 Initial claudication distance (PFWD + COT).
1.7. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 7: ICD (odds ratio)
7.

Forest plot of comparison: 1 Propionyl‐L‐carnitine versus placebo, outcome: 1.8 ICD (odds ratio) with subgroup.
However, large heterogeneity is also present here.
As two trials in this analysis contain only participants with diabetes (Greco 1992; Signorelli 2006a), we investigated whether PLC could have different efficacy in these participants. This small pool with only diabetes participants contained 93 participants and showed a mean relative improvement for ICD of 0.47 (95% CI 0.39 to 0.56) in favor of PLC. In the absence of individual data, the comparator is the pool of all other studies, which contains a mixed group of diabetic and non‐diabetic individuals. This group of the seven remaining studies covered 1058 PP participants and showed a relative improvement of 0.29 (95% CI 0.26 to 0.32) favoring PLC. Differences between subgroups showed a statistically significant difference in treatment effect between subgroups (P < 0.0001) (Analysis 1.7). Obviously, this subgroup analysis using a mixed group as a comparator is not ideal; however this result suggests that PLC could have greater effect in diabetes participants, and this should be further researched.
Quality of life
For people with intermittent claudication (IC), quality of life is an important issue, as pain and the impossibility to walk long distances can severely restrict their daily activities and daily quality of life. In two studies by Brevetti (Brevetti 1995; Brevetti 1999), this item was evaluated. Standardized questionnaires designed to measure quality of life were used to obtain a valuable evaluation. Brevetti 1995 used the McMaster Health Index Questionnaire, containing 59 health‐related statements that investigate the person's psychological, social, and emotional status. Global score improved for PLC participants from 0.59 to 0.64, whereas placebo participants experienced a reduced quality of life, from 0.64 to 0.63. Mean difference was 0.06 (95% CI 0.05 to 0.07) (moderate‐certainty evidence). These results can be found in Analysis 1.11 and in Figure 8.
1.11. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 11: Quality of life (QoL)
8.

Forest plot of comparison: 1 Propionyl‐L‐carnitine versus placebo or control, outcome: 1.11 Quality of life (QoL).
The other study, Brevetti 1999, was not included in this participant pool, as scores are calculated differently from Brevetti 1995, as published in Brevetti 1997; as such, these scores were not convertible. Brevetti 1999 used the questionnaire of Jaeschke and Guyatt to interrogate 114 participants about multiple life domains. In terms of pain and psychological function, a significantly better result was achieved for PLC participants, but for the social function, the difference was not significant.
In light of the two studies, quality of life appears to improve after PLC supplementation. This is related to improved walking performance.
Progression of disease (to Fontaine stage III or IV or necessity for intervention (endovascular or surgery))
PLC efficacy can also be evaluated by the evolution of peripheral arterial disease (PAD). Only one study checked this (Brevetti 1999). Five participants in the PLC group (2%) evolved from Fontaine stage II to stage III (rest pain) versus 10 participants (4%) in the placebo group. However, two PLC participants evolved to stage IV (critical ischemia) versus none of the placebo participants. Larger participant pools and longer follow‐up periods are needed to determine the true difference.
Side effects of the propionyl‐L‐carnitine regimen
In Brevetti 1995, seven adverse events were reported in the placebo group and five adverse events in the PLC group not requiring drug discontinuation. Nausea and gastric pain were the most frequent side effects. In this trial, 11 adverse events resulting in drug discontinuation occurred in the PLC group and three in the placebo group. However, the medical problems requiring drug discontinuation in the PLC group were unrelated to study medication, according to the study authors.
7/127 participants in the control group experienced adverse events.
5/118 participants in the PLC group experienced adverse events.
In Brevetti 1999, 27 PLC participants discontinued the study because of the occurrence of serious adverse events (mainly cardiac and peripheral vascular). In the placebo group, 30 adverse events required study discontinuation. Thirty‐eight adverse events not requiring drug discontinuation occurred in the PLC group, and 98 occurred in the placebo group; flu syndrome was the most frequent adverse effect without a difference of occurrence between arms.
30/246 participants in the control group experienced adverse events requiring study discontinuation (5 cardiac, 2 cerebral, 13 peripheral, 10 for other reasons), and 98/246 participants experienced adverse events not requiring study discontinuation.
27/239 participants in the PLC group experienced adverse events requiring study discontinuation (5 cardiac, 3 cerebral, 12 peripheral, 7 for other reasons), and 38/239 participants experienced adverse events not requiring study discontinuation.
Coto 1992 saw three adverse events requiring study interruption in the PLC group and six in the placebo group. The most common adverse event was abdominal pain.
6/140 participants in the control group experienced adverse events requiring study discontinuation (2 for cardiac reasons, 4 for gastro‐enterological reasons).
3/142 participants in the PLC group experienced adverse events (3 for gastro‐enterological reasons).
Both trials from Hiatt do not mention important differences in side effects between the two intervention arms: 70% of PLC participants and 68% of placebo participants experienced one or more adverse events (Hiatt 2001; Hiatt 2011). In these studies, adverse events that affected more than 5% of participants in either group with a ratio greater than 1.5 PLC versus placebo included nausea, diarrhea, bronchitis, and back pain.
Signorelli 2006b did not find any adverse events in the study population.
The six remaining studies did not report adverse events.
Checking the database of the European Medicines Agency (EMA) and the database of the individual patient experience (DIPEx) registry did not reveal any matched documents.
PLC appears to be a safe and well‐tolerated drug, as no significant differences from placebo can be found in studies with follow‐up from six months to one year.
Ankle brachial index
Four of the included studies investigate the efficacy of PLC for ABI evolution (Greco 1992; Luo 2013; Signorelli 2006a; Signorelli 2006b). Signorelli 2006b focused only on the ABI, as it is the only outcome parameter in this study. The participant pool for this analysis contains 397 participants with an average baseline ABI of 0.69 (placebo group 0.70, PLC group 0.69). There were 28 dropouts, and PP analysis was performed on 369 participants. Results can be found in Analysis 1.12 and Figure 9. Apart from Greco 1992, with a relative weight of only 0.6%, the impact of the other three studies appears more balanced than in ACD and ICD analyses in which domination of one study was found. For PLC participants, ABI has risen 0.06 at the end of the study compared to the baseline value. The control group experienced a negligible difference of 0.007 at the end versus at baseline. In all four studies, the PLC population experienced an increase in mean ABI compared to the baseline ABI. In all but one study, ABI of control participants worsened during follow‐up (Luo 2013). Only in Luo 2013, the control population also showed improvement in ABI. The mean difference between the two intervention arms was 0.09 (95% CI 0.08 to 0.09) (moderate‐certainty evidence), favoring PLC.
1.12. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 12: Ankle brachial index (ABI)
9.

Forest plot of comparison: 1 Propionyl‐L‐carnitine versus placebo or control, outcome: 1.12 Ankle brachial index (ABI).
PLC versus L‐carnitine
Only Brevetti 1992, with a total of 28 participants, has compared the efficacy of PLC and an equimolar dose of L‐carnitine. Investigation of ACD favored PLC supplementation over L‐carnitine (ACD 20.00 m, 95% CI 0.47 to 39.53; 28 participants, 1 study; low‐certainty evidence), with a significance level of 0.04 (see Analysis 2.1). ICD assessment also showed greater improvement among PLC participants, but this difference was not significant (ICD 4.00 m, 95% CI ‐9.86 to 17.86; 28 participants, 1 study; P = 0.57; low‐certainty evidence) (Analysis 2.2).
2.1. Analysis.

Comparison 2: Propionyl‐L‐carnitine versus L‐carnitine, Outcome 1: Maximal walking distance
2.2. Analysis.

Comparison 2: Propionyl‐L‐carnitine versus L‐carnitine, Outcome 2: Initial claudication distance
Brevetti 1992 reported that the ABI index remained unchanged.
Remaining outcomes of the review were not measured by Brevetti 1992.
Subgroup and sensitivity analysis
We performed subgroup analysis according to the participant characteristic diabetes versus non‐diabetes, as described above.
We did not perform subgroup analysis based on route of administration (orally or intravenously) because there were only three iv trials (Andreozzi 2008: PLC versus placebo, outcome: ACD; Signorelli 2006b: PLC versus placebo, outcome: ABI; Brevetti 1992: PLC versus LC, outcome: ICD, ACD and ABI) compared to nine oral PLC trials.
In most trials, dose range was small and was similar at 1 to 2 g/d; therefore we did not perform subgroup analysis based on dose range.
If in future reviews sufficient data are available, we will also carry out subgroup analyses for treatment duration, dose (for propionyl‐L‐carnitine, a wide dose range exists, ranging from 1 g daily to 4 g daily), and route of administration.
We also performed sensitivity analyses including the three studies with the biggest weight in the fixed‐effect model; however effect sizes were similar and heterogeneity did not change.
We did not assess funnel plots because of the insufficient number of included studies.
Discussion
Summary of main results
Individuals with intermittent claudication (IC) should in the first place be treated with conservative interventions such as exercise and management of cardiovascular risk. This Cochrane Review was set up to investigate whether propionyl‐L‐carnitine (PLC) could be an effective adjuvant treatment. All but one of the included studies compared PLC to placebo.
For maximal walking performance (absolute claudication distance ‐ ACD), IC participants on PLC showed 26% more improvement than control participants who received placebo. For pain‐free walking performance (initial claudication distance ‐ ICD), participants on PLC showed 31% more improvement than participants on placebo. These differences between the two intervention arms were significant in absolute distances as well as in relative improvement (as shown using odds ratios (ORs)). For a disease such as IC, the primary goal should be improving functional capacities. Improvement in walking performance can contribute to this by putting less restriction on social and professional activities. Walking outcomes are therefore essential parameters for the IC patient. Two trials showed significant improvement in the outcome parameter quality of life (QoL) with PLC (Brevetti 1995; Brevetti 1999). Ankle brachial index (ABI) increased on PLC therapy and decreased on placebo, showing small but statistical significance. For "progression of disease," evidence was insufficient to permit a clear statement.
In terms of adverse events, overall no remarkable differences were seen between intervention arms. Mainly gastric and intestinal problems were observed, occurring evenly in both arms. Tolerance and safety of this drug appear to be acceptable.
Overall completeness and applicability of evidence
Participants with intermittent claudication who were included in these trials were a representative sample of people with this disease. With a mean baseline ACD of 189 meters, the average participant in this review is at an advanced stage of IC (Fontaine stage IIb), indicating that this group might be the target population for PLC use. Only one study also includes participants with a baseline MWD greater than 250 meters, but the results of this subgroup were not analyzed in the ACD participant pool for this review (Brevetti 1999). Primary and secondary outcomes in this review are relevant parameters for following‐up on the evolution of the disease.
We contacted the company Sigma Tau to ask for potential additional data; the company responded that in relation to our request, there were no supplemental data. On our request, they sent us the publication of Deckert 1997, which refers to the Brevetti 1997 publication, which is an additional publication of the included study Brevetti 1995.
Only one small study compared PLC with treatments other than placebo or control: PLC versus L‐carnitine (Brevetti 1992).
Most studies do not report on the outcomes QoL, progression of disease, side effects, and ABI.
Although all results revealed significant differences, a pronounced placebo effect of over 50% was often observed. This phenomenon could be attributed to a tendency towards improved exercise tolerance on repeated examinations (training effect). Moreover, it is conceivable that an outcome such as walking performance is strongly influenced by participants' enhanced motivation in response to the intense follow‐up (Hawthorne effect). Anyhow, confounding factors are hard to fully avoid when studying and following up on patients.
Generally, in studies on treatment for IC, there is a large range of treatment durations ‐ from four days to one year. Trials on treatment in IC recommend treatment duration of at least three months. In this review, treatment duration of the included studies was between six months and one year.
Based on current price indications, PLC supplementation of 1 to 2 g/d would cost around one Euro a day.
Quality of the evidence
For this review, we included only double‐blind randomized controlled trials. Most studies were double‐blind ‐ for one study, this is not clearly stated but the double‐blind character is assumed (Andreozzi 2008). Studies had to fulfill quality criteria and underwent a detailed risk of bias assessment; studies were included in the review only if none of the risk of bias domains were judged to be at high risk of bias.
With all data taken into account, the overall certainty of evidence for the comparison between PLC and placebo/control is considered moderate. There is heterogeneity due to study size (some studies have large participant numbers (282); other trials include only 19 participants), participants coming from different countries and centers, the combination of participants with and without diabetes, and use of different treadmill protocols. To cope with these differences in characteristics of participant pools, weight was given to each trial. Outcomes were similar in all studies and were assessed by standardized testing methods. To deal with differences in treadmill speed and slope and a wide range of baseline values, relative improvements were also calculated (odds ratios) and analyzed.
Besides clinical heterogeneity, there is also substantial statistical heterogeneity throughout the current review. To deal with this heterogeneity, we performed similar analyses using standardized effect size. However, heterogeneity even increased with this approach. Furthermore, we performed the same analyses with a random‐effects model (instead of the fixed‐effect model), but overall results were largely similar to those of the fixed‐effect model, as well as similar heterogeneity for both approaches. Due to the number of smaller studies, a random‐effects model is not the optimal solution, which is why we opted for the fixed‐effect model. Finally, post‐hoc sensitivity analyses, which retained only the three studies contributing the largest weight to the fixed‐effect model, had no effect on reducing or explaining this heterogeneity.
The comparison between PLC and L‐carnitine in a single study with few participants and a short follow‐up period should be interpreted with caution (Brevetti 1992). We judged the certainty of evidence to be low.
Potential biases in the review process
The studies included in this review generally have low to moderate participant numbers. In ACD and ICD analyses, nine included studies created participant pools of over 1000 participants. This should be sufficient to generate a reliable outcome when the efficacy of the study drug is evaluated.
As already mentioned, the weight of studies was calculated according to participant numbers and standard deviations. In all analyses, Brevetti 1995 has a major impact up to 80%, leaving the other large trials of minor weight. It can then be questioned whether the results of Brevetti 1995 are indeed reliable, and if there was no selective reporting or participant recruitment. However, after assessment, overall this study seems to be well conducted. We performed a sensitivity analysis by excluding Brevetti 1995. This did not change the overall outcome. After Brevetti 1995 was excluded, a moderate although statistically significant difference of 55 meters (versus 50 meters) for ACD and of 39 meters (versus 33 meters) for ICD remained.
All time measurements from PWT studies were converted to absolute distances (in meters), according to treadmill testing speed. This might be subject to discussion, given the fact that for example studies that use PWT on a graded treadmill are not comparable to those using MWD on a constant‐load treadmill. However, different treadmill protocols exist within studies measuring MWD and within those measuring PWT. Therefore, we also based the data on the treatment effect of the ratio (or per cent change) for the benefit of propionyl‐L‐carnitine relative to the benefit of placebo or control (or other active) treatment. For Brevetti 1999, only the S1 group (variability < 25% and MWD < 250 m) was analyzed.
Agreements and disagreements with other studies or reviews
Results from this Cochrane Review are similar to the results of the Brass 2013 review on the same topic. Most of the studies used in Brass 2013 were also included in the present review (Brass also included the study of Strano 2002, which was excluded from this review). Delaney 2013 observed the same trend in a review comparing carnitine derivatives with placebo, favoring carnitine derivatives over placebo; however, results are less specific for PLC.
Authors' conclusions
Implications for practice.
For symptomatic PAD, treatment includes maximal risk modification, exercise, and, when indicated, invasive treatment consisting of balloon angioplasty with or without stenting and/or bypass surgery. Additionally, several pharmacological substances have been tested to determine if they improve walking capacity. The efficacy of many of these drugs is controversial. Based on results of this review, PLC appears to have some potential in the treatment of IC in the studied population. The participant pool from this review, with average baseline ACD, ICD, and ABI of 189 meters, 120 meters, and 0.69 meters, respectively, is already in an advanced stage of IC (Fontaine class IIb). One study tested participants with a baseline ACD greater than 250 meters, but PLC was not effective in this population (Brevetti 1999). So, treatment with PLC in clinical practice might be considered for patients with IC with a rather advanced limitation of their walking performance when classic treatments are not sufficient or feasible. The increase in ABI was statistically significant, but the net effect size is probably too small to detect differences in clinical practice.
PLC has no place in standard treatment. PLC treatment might be considered in practice adjuvant to exercise and other conservative interventions, when these seem insufficient for symptom relief. In cases where endovascular procedures or invasive surgery is not indicated or contraindicated, PLC might be considered for symptomatic treatment on a patient‐by‐patient basis; however no RCT evidence was found comparing PLC with standard treatment to directly support such use.
In terms of adverse events, overall, no remarkable differences were seen between intervention arms. Mainly gastric and intestinal problems were observed, occurring evenly in all arms. The tolerance and safety of this drug appear to be acceptable. PLC could be given to IC patients as try‐out medicine. When the intervention does not bring improvement in symptoms after a certain period (e.g. three to six months), it can easily be stopped. It should be emphasized that this review did not assess the effectiveness of PLC for mortality or other hard endpoints, and there is no evidence that it slows down the evolution of PAD. PLC may be considered only potential symptomatic treatment to improve quality of life by improving walking performance.
Implications for research.
In the past 25 years, several PLC trials on people with IC have been conducted. In these studies, outcomes were mainly the primary and secondary outcomes of this review (ACD, ICD, ABI). Future trials should systematically include a robust quality of life assessment. Walking performances are essential in the evaluation of IC; however evidence could be stronger if psychosocial effects and the global well‐being of a drug intervention are also evaluated. Following up on "fate of the claudicant limb" in longitudinal studies and cost‐effectiveness of the drug treatment, whoever is the payer, could also be useful outcomes.
All but one of the studies included in this review were studies on the efficacy of PLC versus placebo. More research with this medication could be done, such as head‐to‐head comparisons with other vasoactive drugs.
History
Protocol first published: Issue 9, 2012
Acknowledgements
We thank Dr Marlène Stewart for co‐ordinating the editorial process and for providing support in the course of the review process. We thank the Cochrane Vascular Group. We thank Mrs Heather Maxwell, Professor William Hiatt, and Dr Andrew Herxheimer for their feedback and advice in the peer review process for the protocol of this systematic review. We thank Dr Karen Welch for co‐ordinating the trials search and for providing support in the course of the review process. We thank Prof Dr Andreozzi for sending us additional data from his trial. We thank Dr Vincenzo Giordano for responding to our questions.
We are grateful to the following peer reviewers for their time and comments: Mr Harvey Chant, MD, FRCS (Royal Cornwall Hospital, UK) and the peer reviewer who wished to remain anonymous.
Appendices
Appendix 1. Abbreviations
ABI: ankle brachial index ACD: absolute claudication distance ATP: adenosine‐5'‐triphosphate CoA: coenzyme A COT: claudication onset time IC: intermittent claudication ICD: initial claudication distance ITT: intention‐to‐treat (population) iv: intravenously LC: L(evo)‐carnitine MWD: maximal walking distance PAD: peripheral arterial disease PFWD: pain‐free walking distance PLC: propionyl‐L(evo)‐carnitine PP: per‐protocol (population) PTA: percutaneous transluminal angioplasty PWT: peak walking time RCT: randomized controlled trial
Appendix 2. CENTRAL search strategy, March 2017
| #1 | MESH DESCRIPTOR Arteriosclerosis | 869 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 72 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 645 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 737 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 726 |
| #7 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2236 |
| #8 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9508 |
| #9 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 8384 |
| #10 | (peripheral near3 dis*):TI,AB,KY | 3533 |
| #11 | (claudic* or IC):TI,AB,KY | 3229 |
| #12 | dysvascular*:TI,AB,KY | 11 |
| #13 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 99 |
| #14 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 158 |
| #15 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 82 |
| #16 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1113 |
| #17 | MESH DESCRIPTOR Iliac Artery | 147 |
| #18 | MESH DESCRIPTOR Popliteal Artery | 282 |
| #19 | MESH DESCRIPTOR Femoral Artery | 834 |
| #20 | MESH DESCRIPTOR Tibial Arteries | 33 |
| #21 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 1220 |
| #22 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 or #21 | 24366 |
| #23 | MESH DESCRIPTOR Carnitine | 438 |
| #24 | *propionyl*:TI,AB,KY | 128 |
| #25 | *carnitin*:TI,KY,AB | 1115 |
| #26 | #23 OR #24 OR #25 | 1163 |
| #27 | #22 AND #26 | 63 |
Appendix 3. Search strategies, last searched July 2021
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW | Carnitine | June 5, 2018: 0 Oct 21, 2019: 37 July 7, 2021: 68 |
| CENTRAL | #1 MESH DESCRIPTOR Arteriosclerosis 954 #2 MESH DESCRIPTOR Arteriolosclerosis 0 #3 MESH DESCRIPTOR Arteriosclerosis Obliterans 84 #4 MESH DESCRIPTOR Atherosclerosis 1148 #5 MESH DESCRIPTOR Arterial Occlusive Diseases 839 #6 MESH DESCRIPTOR Intermittent Claudication 854 #7 MESH DESCRIPTOR Ischemia 1687 #8 MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES 2915 #9 MESH DESCRIPTOR Vascular Diseases 693 #10 atherosclero* or arteriosclero* or PVD or PAOD or PAD 29412 #11 ((arter*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*)):TI,AB,KY 5722 #12 ((vein*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) ):TI,AB,KY 424 #13 ((veno*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) ):TI,AB,KY 314 #14 ((peripher*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) ):TI,AB,KY 1542 #15 (peripheral near/3 dis* ):TI,AB,KY 0 #16 arteriopathic:TI,AB,KY 7 #17 (claudic* or hinken*):TI,AB,KY 2304 #18 (isch* or CLI):TI,AB,KY 41053 #19 dysvascular*:TI,AB,KY 26 #20 (leg near4 (obstruct* or occlus* or steno* or block* or obliter*) ):TI,AB,KY 181 #21 (limb near4 (obstruct* or occlus* or steno* or block* or obliter*) ):TI,AB,KY 316 #22 ((vascular) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) ):TI,AB,KY 689 #23 ((lower near3 extrem*) near4 (obstruct* or occlus* or steno* or block* or obliter*) ):TI,AB,KY 134 #24 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 74911 #25 MESH DESCRIPTOR Carnitine EXPLODE ALL TREES 590 #26 propionyl*:TI,AB,KY 125 #27 carnitin*:TI,AB,KY 1606 #28 #25 OR #26 OR #27 1654 #29 #24 AND #28 147 #30 01/01/2018 TO 21/10/2019:CD 504644 #31 #29 AND #30 33 |
June 5, 2018: 20 Oct 21, 2019: 33 July 12, 2021: 50 |
| Clinicaltrials.gov | intermittent claudication OR Peripheral Vascular Diseases OR Arteriosclerosis | Carnitine OR Propionyl‐L‐carnitine OR L‐Carnitine | Last update posted on or before 06/05/2018 | June 5, 2018: 9 Oct 21, 2019: 0 July 12, 2021: 0 |
| ICTRP Search Portal | intermittent claudication OR Peripheral Vascular Diseases OR Arteriosclerosis | Carnitine OR Propionyl‐L‐carnitine OR L‐Carnitine | 01/01/2017‐ 06/05/2018 | June 5, 2018: 0 Oct 21, 2019: 1 July 12, 2021: 0 |
| MEDLINE | 1 ARTERIOSCLEROSIS/ 56444 2 ARTERIOLOSCLEROSIS/ 150 3 Arteriosclerosis Obliterans/ 3973 4 ATHEROSCLEROSIS/ 30905 5 Arterial Occlusive Diseases/ 26463 6 Intermittent Claudication/ 7584 7 ISCHEMIA/ 47448 8 exp Peripheral Vascular Diseases/ 49936 9 Vascular Diseases/ 34879 10 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 170890 11 (arter* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 64720 12 (vascular adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 16651 13 (vein* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 8133 14 (veno* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 9619 15 (peripher* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 2090 16 (peripheral adj3 dis*).ti,ab. 37690 17 arteriopathic.ti,ab. 162 18 (claudic* or hinken*).ti,ab. 9799 19 (isch* or CLI).ti,ab. 345087 20 dysvascular*.ti,ab. 216 21 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 587 22 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 1813 23 (lower adj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 1535 24 or/1‐23 698093 25 exp CARNITINE/ 9044 26 propionyl*.ti,ab. 3452 27 carnitin*.ti,ab. 13550 28 Bicarnesine.ti,ab. 7 29 L‐Carnitine.ti,ab. 4381 30 Levocarnitine.ti,ab. 114 31 "Vitamin BT".ti,ab. 12 32 or/25‐31 18429 33 24 and 32 1020 34 randomized controlled trial.pt. 463116 35 controlled clinical trial.pt. 92464 36 randomized.ab. 414463 37 placebo.ab. 189857 38 drug therapy.fs. 2025083 39 randomly.ab. 292191 40 trial.ab. 431325 41 groups.ab. 1805152 42 or/34‐41 4224209 43 33 and 42 456 44 (2017* or 2018*).ed. 1387224 45 43 and 44 24 46 from 45 keep 1‐24 24 |
June 5, 2018: 24 Oct 21, 2019: 23 July 12, 2021: 31 |
| Embase | 1 arteriosclerosis/ 33940 2 arteriolosclerosis/ 594 3 peripheral occlusive artery disease/ 33087 4 atherosclerosis/ 135761 5 intermittent claudication/ 9743 6 ischemia/ 76455 7 exp peripheral vascular disease/ 1653544 8 vascular disease/ 60113 9 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. 235430 10 (arter* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 88098 11 (vascular adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 22360 12 (vein* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 11390 13 (veno* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 12776 14 (peripher* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. 3170 15 (peripheral adj3 dis*).ti,ab. 54106 16 arteriopathic.ti,ab. 205 17 (claudic* or hinken*).ti,ab. 13192 18 (isch* or CLI).ti,ab. 498517 19 dysvascular*.ti,ab. 237 20 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 779 21 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 2714 22 (lower adj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 1951 23 or/1‐22 1948127 24 exp carnitine/ 14322 25 propionyl*.ti,ab. 3957 26 carnitin*.ti,ab. 17103 27 Bicarnesine.ti,ab. 7 28 L‐Carnitine.ti,ab. 5675 29 Levocarnitine.ti,ab. 198 30 "Vitamin BT".ti,ab. 13 31 or/24‐30 25156 32 randomized controlled trial/ 504919 33 controlled clinical trial/ 460994 34 random$.ti,ab. 1308003 35 randomization/ 78329 36 intermethod comparison/ 235372 37 placebo.ti,ab. 273256 38 (compare or compared or comparison).ti. 469523 39 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 1750381 40 (open adj label).ti,ab. 64476 41 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 208948 42 double blind procedure/ 150474 43 parallel group$1.ti,ab. 21802 44 (crossover or cross over).ti,ab. 92895 45 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 282700 46 (assigned or allocated).ti,ab. 331640 47 (controlled adj7 (study or design or trial)).ti,ab. 294772 48 (volunteer or volunteers).ti,ab. 224372 49 or/32‐48 3981021 50 31 and 49 3464 51 (2017* or 2018*).dc. 2550592 52 50 and 51 431 53 from 52 keep 1‐431 431 |
June 5, 2018: 431 Oct 21, 2019: 617 July 12, 2021: 900 |
| CINAHL | S46 S44 AND S45 2 S45 EM 2017 OR EM 2018 359,649 S44 S22 AND S30 AND S43 25 S43 S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 340,984 S42 MH "Random Assignment" 38,485 S41 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" 32,686 S40 MH "Crossover Design" 11,182 S39 MH "Factorial Design" 919 S38 MH "Placebos" 8,348 S37 MH "Clinical Trials" 93,080 S36 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" 4,461 S35 TX crossover OR "cross‐over" 14,520 S34 AB placebo* 28,198 S33 TX random* 218,248 S32 TX trial* 249,585 S31 TX "latin square" 142 S30 S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 1,012 S29 TX Vitamin BT 1 S28 TX Levocarnitine 20 S27 TX L‐Carnitine 302 S26 TX Bicarnesine 0 S25 TX carnitin* 994 S24 TX propionyl* 43 S23 (MH "Carnitine") 672 S22 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 79,837 S21 TX (lower n3 extrem*) n4 (obstruct* or occlus* or steno* or block* or obliter*) 85 S20 TX limb n4 (obstruct* or occlus* or steno* or block* or obliter*) 198 S19 TX leg n4 (obstruct* or occlus* or steno* or block* or obliter*) 92 S18 TX dysvascular* 172 S17 TX isch* or CLI 39,325 S16 TX claudic* or hinken* 1,401 S15 TX arteriopathic 10 S14 TX peripheral n3 dis* 9,236 S13 TX peripher*) n (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) 11 S12 TX (veno*) n (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) 12 S11 TX (vein*) n (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) 8 S10 TX (vascular) n (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) 13 S9 TX (arter*) n (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) 170 S8 TX atherosclero* or arteriosclero* or PVD or PAOD or PAD 26,324 S7 (MH "Vascular Diseases") 2,414 S6 (MH "Peripheral Vascular Diseases+") 10,389 S5 (MH "Ischemia") 3,365 S4 (MH "Intermittent Claudication") 852 S3 (MH "Arterial Occlusive Diseases") 1,607 S2 (MH "Atherosclerosis") 3,313 S1 (MH "Arteriosclerosis") 4,829 |
June 5, 2018: 2 Oct 21, 2019: 4 July 12, 2021: 5 |
| AMED | 1 ARTERIOSCLEROSIS/ 78 2 ATHEROSCLEROSIS/ 221 3 Intermittent Claudication/ 73 4 ISCHEMIA/ 263 5 [(arter* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab.] 0 6 [(vascular adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab.] 0 7 [(vein* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab.] 0 8 [(veno* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab.] 0 9 [(peripher* adj (*occlus*/ or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab.] 0 10 (peripheral adj3 dis*).ti,ab. 435 11 arteriopathic.ti,ab. 1 12 (claudic* or hinken*).ti,ab. 148 13 (isch* or CLI).ti,ab. 1666 14 dysvascular*.ti,ab. 57 15 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 19 16 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 22 17 (lower adj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. 12 18 exp CARNITINE/ 17 19 propionyl*.ti,ab. 8 20 carnitin*.ti,ab. 87 21 Bicarnesine.ti,ab. 0 22 L‐Carnitine.ti,ab. 45 23 Levocarnitine.ti,ab. 1 24 "Vitamin BT".ti,ab. 0 25 or/1‐17 2540 26 or/18‐24 96 27 25 and 26 4 |
June 5, 2018: 0 Oct 21, 2019: 1 July 12, 2021: 0 |
Data and analyses
Comparison 1. Propionyl‐L‐carnitine versus placebo or control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Absolute claudication distance (MWD + PWT) | 9 | 1121 | Mean Difference (IV, Fixed, 95% CI) | 50.86 [50.34, 51.38] |
| 1.2 ACD (odds ratio) | 9 | 1121 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.23, 0.28] |
| 1.3 Maximal walking distance (MWD) | 6 | 692 | Mean Difference (IV, Fixed, 95% CI) | 50.43 [49.91, 50.96] |
| 1.4 Peak walking time (PWT) | 3 | 429 | Mean Difference (IV, Fixed, 95% CI) | 84.04 [79.57, 88.51] |
| 1.5 ACD without Brevetti 1995 (sensitivity analysis) | 8 | 907 | Mean Difference (IV, Fixed, 95% CI) | 55.90 [54.85, 56.95] |
| 1.6 Initial claudication distance (PFWD + COT) | 9 | 1151 | Mean Difference (IV, Fixed, 95% CI) | 32.98 [32.60, 33.37] |
| 1.7 ICD (odds ratio) | 9 | 1151 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [0.28, 0.34] |
| 1.7.1 Mixed group of diabetic and non‐diabetic patients | 7 | 1058 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.26, 0.32] |
| 1.7.2 Diabetic patients only | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.39, 0.56] |
| 1.8 Pain‐free walking distance (PFWD) | 6 | 722 | Mean Difference (IV, Fixed, 95% CI) | 33.19 [32.80, 33.58] |
| 1.9 Claudication onset time (COT) | 3 | 429 | Mean Difference (IV, Fixed, 95% CI) | 27.76 [25.05, 30.48] |
| 1.10 ICD without Brevetti 1995 (sensitivity analysis) | 8 | 937 | Mean Difference (IV, Fixed, 95% CI) | 39.24 [38.52, 39.96] |
| 1.11 Quality of life (QoL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12 Ankle brachial index (ABI) | 4 | 369 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [0.08, 0.09] |
1.3. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 3: Maximal walking distance (MWD)
1.4. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 4: Peak walking time (PWT)
1.5. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 5: ACD without Brevetti 1995 (sensitivity analysis)
1.8. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 8: Pain‐free walking distance (PFWD)
1.9. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 9: Claudication onset time (COT)
1.10. Analysis.

Comparison 1: Propionyl‐L‐carnitine versus placebo or control, Outcome 10: ICD without Brevetti 1995 (sensitivity analysis)
Comparison 2. Propionyl‐L‐carnitine versus L‐carnitine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Maximal walking distance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Initial claudication distance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andreozzi 2008.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled trial | |
| Participants | 44 male participants (22 intervention, 22 control) with intermittent claudication, admitted to the day hospital of the clinic of the authors. Half of the participants suffered from severe claudication (MWD < 100 m) and half had moderate claudication (MWD < 200 m), resulting in 4 equal subgroups of 11 participants each, based on intervention/control and severity of claudication | |
| Interventions | Intervention: physical training 3 times weekly for 6 weeks + intravenous saline solution + intravenous 600 mg propionyl‐L‐carnitine 3 times weekly during the last 3 weeks of training Control: physical training 3 times weekly for 6 weeks + intravenous saline solution 3 times weekly during the last 3 weeks of training |
|
| Outcomes | Absolute claudication distance (= maximal walking distance) on treadmill (speed 2.5 km/h, slope 15%). Measurements were performed at baseline and after 3 and 6 weeks | |
| Notes | Disease specific: rather short study duration Study authors did not receive any funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation of parallel groups |
| Allocation concealment (selection bias) | Unclear risk | No description of the method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information about blinding available; double‐blind character assumed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information to permit judgement of "low risk" or "high risk" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All results for groups and subgroups are reported; all P values are calculated. No withdrawals from the study reported. Results for the whole population (severe + moderate) were requested and were received from the study author |
| Selective reporting (reporting bias) | Low risk | Extensive reporting on ACD; probably no reporting bias |
| Other bias | Unclear risk | Disease specific: rather short study duration |
Brevetti 1992.
| Study characteristics | ||
| Methods | Randomized double‐blind controlled trial; cross‐over study | |
| Participants | 12 intermittent claudication patients in the preliminary study (dose‐finding study), 14 intermittent claudication patients in the comparative study (iv 600 mg PLC vs iv 500 mg LC). Patients were referred to the outpatient clinic of the study authors | |
| Interventions | Preliminary study: first iv placebo for all participants, 4 days later followed by intervention: iv 300 mg PLC or 600 mg PLC (cross‐over after 4 days between the 2 PLC dose arms) Comparative study: first iv placebo for all participants, 4 days later followed by intervention: iv 600 mg PLC iv or 500 mg LC (cross‐over after 4 days between the 2 intervention arms) There was a washout period between the different phases |
|
| Outcomes | Maximal walking distance (meters) on treadmill (2.5 mph, slope 7%) Initial (pain‐free) claudication distance (meters) on treadmill (2.5 mph, slope 7%) Hemodynamic assessment: CSA common femoral artery, blood flow velocity (cm/s), blood flow rate (mL/min), pulsatility index, resistance index, ABI |
|
| Notes | Quote in the discussion: "an increase in MWD of 30% was accepted as clinically relevant" Disease specific: short study duration and follow‐up Small number of participants No washout period in the comparative study Funding by Sigma Tau |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about random generation of the sequence of phases and groups (cross‐over study) |
| Allocation concealment (selection bias) | Unclear risk | No description of the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, double‐dummy cross‐over study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All results and P values reported; no reported withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Quote in the discussion: "only an increase in MWD of 30% over baseline was accepted as clinically relevant": 8/14 on PLC and 5/14 on LC. It is not fully clear which numbers were used for analysis |
| Other bias | Unclear risk | Possible conflict of interest (funding by Sigma Tau), short duration and follow‐up, small number of participants |
Brevetti 1995.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind trial with a dose‐titration design adopted by 13 centers for the study | |
| Participants | 245 (ITT) ‐ 214 (PP) patients with intermittent claudication. Patients underwent a 2‐week washout phase in which they were familiarized with the treadmill. 31 dropouts were reported (14 due to various adverse events and 17 due to poor compliance) | |
| Interventions | Intervention (n = 99): oral PLC 1/2/3 g/d for 6 months Control (n = 115): oral placebo 1//2/3 g/d for 6 months Initial dose of 2 × 500 mg daily was increased at 2‐month intervals to 2 g daily, then to 3 g daily, in participants with improvement in treadmill performance < 30% over baseline; participants showing improvement > 30% continued with the same dose as in the previous 2 months |
|
| Outcomes | Maximal walking distance on treadmill (speed 4 km/h, slope 7%) Initial claudication distance (PFWD) on treadmill (speed 4 km/h, slope 7%) Analysis of titration course: probability of obtaining an increase in MWD ≥ 30% with a specific dose |
|
| Notes | Funding by Sigma Tau | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation of parallel groups |
| Allocation concealment (selection bias) | Unclear risk | No description of method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout data (19 PLC group, 12 placebo group) are presented; all data for all main outcomes and groups reported |
| Selective reporting (reporting bias) | Unclear risk | Main analysis was not split into subgroups, as was done in the additional publication ‐ Brevetti 1997 |
| Other bias | Unclear risk | Comment: main analysis was not split into subgroups for severe and moderate claudication, as was done in additional publication ‐ Brevetti 1997 ‐ for the same population
Pronounced outcome discrepancy between groups A (98 patients coming from 1 center) and B (116 patients coming from 12 centers) Quote: "a marked center effect was observed because the 77 patients studied in 1 center were more severely affected than those studied in the remaining 12 centers" Possible conflict of interest (support from Sigma Tau) |
Brevetti 1999.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind trial | |
| Participants | 485 (ITT) ‐ 328 (PP) patients with intermittent claudication minimum 1 year. Patients were stratified into 4 groups (S1 to S4) on the basis of maximal walking distance at baseline (cutoff point 250 m) and maximal walking distance variability at baseline (cutoff point 25%). 485 patients were considered in the intention‐to‐treat protocol; 328 completed the 1‐year protocol Patients taking < 75% of prescribed dose were considered as dropouts 157 dropouts were observed: 10 participants died; 57 dropped out due to adverse events, 61 because of protocol violations; 29 participants were lost to follow‐up. Dropout ratios were balanced between the 2 treatment arms |
|
| Interventions | Intervention: oral PLC (n = 162) 1 g twice daily for 1 year Control: oral placebo (n = 166) 1 g twice daily for 1 year |
|
| Outcomes | Maximal walking distance on treadmill (slope 7%, speed 3 km/h) Initial claudication distance (PFWD) on treadmill (slope 7%, speed 3 km/h) Quality of life Adverse events Measurements were performed every 2 months |
|
| Notes | Funding by Sigma Tau | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation of parallel groups |
| Allocation concealment (selection bias) | Unclear risk | No description of method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind, placebo‐controlled, parallel design" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout data available; all data for groups and subgroups reported and ITT/PP analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Selective focus on significant results (text, figures, subgroups) in S1 and S2 populations; minor reporting on S3 and S4 populations, in whom no significant difference between treatments was observed |
| Other bias | Unclear risk | Possible conflict of interest (funding by Sigma Tau) |
Coto 1992.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind trial with patients from 7 centers | |
| Participants | 300 (ITT) ‐ 282 (PP) patients with intermittent claudication minimum 1 year, randomized in 2 groups. 18 dropout patients (due to adverse events or poor collaboration) | |
| Interventions | Intervention (n = 140): oral PLC 2 × 1 g/d for 6 months Control (n = 142): oral placebo 2 × 1 g/d for 6 months |
|
| Outcomes | Absolute claudication distance (= maximal walking distance) on treadmill (speed 3 km/h, slope 7%) Initial claudication distance (= pain‐free walking distance) on treadmill (speed 3 km/h, slope 7%) Adverse effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation of parallel groups |
| Allocation concealment (selection bias) | Unclear risk | No description of method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a randomized double blind technique for parallel groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all outcomes and all subgroups available; dropout data reported |
| Selective reporting (reporting bias) | Unclear risk | Main outcome reported coefficient of regression as comparator + P values. May be less clinically relevant |
| Other bias | Low risk | No other concerns; however, no statement of conflict of interest |
Dal Lago 1999.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind trial | |
| Participants | 22 (ITT) ‐ 19 (PP) patients with intermittent claudication minimum 1 year, MWD between 150 and 400 m, and ABI < 0.80 | |
| Interventions | Intervention: oral PLC 1 g/d during 90 days Control: oral placebo 1 g/d during 90 days |
|
| Outcomes | Absolute claudication distance (= maximal walking distance) on treadmill (speed 4 km/h, slope 4%) Relative claudication distance (= initial claudication distance or pain‐free walking distance) on treadmill (speed 4 km/h, slope 4%) Doppler spectral analysis for blood flow |
|
| Notes | Small number of participants. No funding reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the patients were assigned to two treatment groups using the procedure of simple randomization according to Pocock" (minimization) |
| Allocation concealment (selection bias) | Unclear risk | No description of method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout data available; all data and P values for outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Study author reports variability between and within groups, but no detailed data on this are reported |
| Other bias | Unclear risk | Small number of participants |
Greco 1992.
| Study characteristics | ||
| Methods | Randomized controlled double‐blind trial | |
| Participants | 20 diabetic patients with intermittent claudication minimum 1 year, ABI < 0.75, MWD from 100 to 500 m, and < 20% variation in ACD during the washout period. 20 patients were randomized into 2 groups of 10 participants. 1 dropout from the placebo group was reported | |
| Interventions | Intervention (n = 10): oral PLC 1.5 g/d for 6 months Control (n = 10): oral placebo 1.5 g/d for 6 months |
|
| Outcomes | Initial claudication distance (PFWD) on treadmill (slope 10%, speed 2.5 km/h) Absolute claudication distance (MWD) on treadmill Ankle brachial Index |
|
| Notes | Funding by Sigma Tau | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation of parallel groups |
| Allocation concealment (selection bias) | Unclear risk | No description of method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a controlled double‐blind trial versus placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout data available; all results reported in absolute and relative numbers; P values reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias assumed |
| Other bias | Unclear risk | Small number of participants (20); possible conflict of interest (funding by Sigma Tau) |
Hiatt 2001.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind trial | |
| Participants | 161 (ITT) ‐ 155 (PP) patients with intermittent claudication recruited from 10 centers in USA (6) and Russia (4). There were 6 dropouts due to loss to follow‐up | |
| Interventions | Intervention (n = 82): oral PLC 2 g/d for 6 months Control (n = 73): oral placebo 2 g/d for 6 months |
|
| Outcomes | Peak walking time on treadmill (speed 2 mph, slope 12%) Claudication onset time on treadmill (speed 2 mph, slope 12%) |
|
| Notes | Funding by Sigma Tau. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were then (after a 2‐week placebo run‐in period) randomly assigned to the placebo or propionyl‐L‐carnitine groups, using a computer‐based algorithm, with balanced blocks of subjects" |
| Allocation concealment (selection bias) | Unclear risk | No description of method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" Study authors state that differences in outcomes between Russian and US subjects could not be accounted for by unblinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout data available; all data and P values for outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Differences between US and Russian centers extensively described. Differences between US and Russian outcome data attributed to differences in baseline population characteristics. Main outcome data (walking times) given only for the whole population |
| Other bias | Unclear risk | Possible conflict of interest (funding by Sigma Tau) |
Hiatt 2011.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind multi‐center trial | |
| Participants | 69 patients between 40 and 80 years with intermittent claudication minimum 1 year and peak walking time between 90 and 360 seconds at baseline were randomized (modified ITT analysis on 62 patients who got at least 1 post‐randomization treadmill test; 7 dropouts due to adverse events and study withdrawal) | |
| Interventions | Intervention (n = 32): oral PLC 2 g/d + instruction on home‐based physical exercise 3 times weekly; duration 6 months Control (n = 30): oral placebo 2 g/d + instruction on home‐based physical exercise 3 times weekly; duration 6 months |
|
| Outcomes | Peak walking time on treadmill (speed 2 mph, slope increase 2%/2 min) Claudication onset time on treadmill (speed 2 mph, slope increase 2%/2 min) |
|
| Notes | Funding by Sigma Tau Home‐based physical exercise checked with a Stepwatch activity monitor |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation of parallel groups. A significant difference in proportion of diabetic patients in the subgroups was observed: fewer patients with diabetes were randomized to PLC |
| Allocation concealment (selection bias) | Unclear risk | No description of method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment + several tests during double‐blind period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout data available; all data and P values for outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | Limitations reported by study authors: inaccurate reporting by participants on physical exercise may lead to bias in analysis |
| Other bias | Unclear risk | Possible conflict of interest (funding by Sigma Tau) Quote: "there were fewer patients with diabetes randomized to PLC than placebo, P = 0.029" |
Luo 2013.
| Study characteristics | ||
| Methods | Randomized multicenter phase 3 double‐blind parallel‐group study | |
| Participants | 239 (full analysis set: all patients with at least 1 post‐baseline assessment) ‐ 212 (per‐protocol set: all patients who completed the trial; dropouts due to adverse events in both groups) patients with intermittent claudication with baseline MWD between 50 and 250 meters and baseline ABI < 0.90 | |
| Interventions | Intervention: oral PLC 2 g/d for 4 months (n = 103) Control: oral placebo 2 g/d for 4 months (n = 109) |
|
| Outcomes | Peak walking time (PWT) Claudication onset time (COT) ABI Treadmill speed and slope not given; speed of 2 mph is assumed (as used in eligibility tests for MWD) |
|
| Notes | Financial sponsoring from Lee’s pharmaceutical holding limited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation of parallel groups |
| Allocation concealment (selection bias) | Unclear risk | No description of method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results are complete and analyses performed for both FAS and PPS groups. Exact adverse events as reasons for dropout are missing |
| Selective reporting (reporting bias) | Low risk | No selective reporting concerns |
| Other bias | Low risk | Quote: "no conflict of interest statement" |
Signorelli 2006a.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind clinical trial | |
| Participants | 74 patients with NIDDM‐associated PAD (stage 2 Leriche classification) | |
| Interventions | Intervention (n = 37): oral PLC 2 g/d for 1 year Control (n = 37): oral placebo 2 g/d for 1 year |
|
| Outcomes | ABI with Doppler Pain‐free walking distance (= initial claudication distance) on treadmill (3.5 km/h, 7.5%) Measurements at baseline and at 6 and 12 months |
|
| Notes | No funding reported No MWD reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned to one of two groups according to a simple randomisation scheme" |
| Allocation concealment (selection bias) | Unclear risk | No description of method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts; all data and P values reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias assumed; no MWD reported (not known if measured) |
| Other bias | Low risk | No further concerns; however, no statement on conflict of interest |
Signorelli 2006b.
| Study characteristics | ||
| Methods | Randomized placebo‐controlled double‐blind clinical trial | |
| Participants | 64 patients with intermittent claudication on hemodialysis (chronic kidney insufficiency) No dropouts were reported | |
| Interventions | Intervention (32): PLC 600 mg iv in saline solution 3 times/week during 1 year Control (32): placebo: only saline solution infusion 3 times/week during 1 year | |
| Outcomes | ABI by continuous Doppler pulse wave Plasma MDA Plasma 4‐HNE Plasma nitrite/nitrate Measurements at baseline and at 6 and 12 months | |
| Notes | No walking distances reported in this study No funding reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a simple randomisation scheme, patients were assigned to one of two groups" |
| Allocation concealment (selection bias) | Unclear risk | No description of method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment assumed, hence no influence on outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts; all data reported (ABI) |
| Selective reporting (reporting bias) | Low risk | No selective reporting concerns |
| Other bias | Low risk | No further concerns; however, no statement on conflict of interest |
4‐HNE: 4‐hydroxynonenal (biomarker of oxidative stress and lipid peroxidation). ABI: ankle brachial index. ACD: absolute claudication distance. COT: claudication onset time. CSA: cross‐sectional area. FAS: full analysis set. ITT: intention‐to‐treat (population). iv: intravenously. LC: L(evo)‐carnitine. MDA: malondialdehyde (indicators of lipid peroxidation). MWD: maximal walking distance. NIDDM: non‐insulin‐dependent diabetes mellitus. PAD: peripheral arterial disease. PFWD: pain‐free walking distance. PLC: propionyl‐L(evo)‐carnitine. PP: per‐protocol (population). PPS: per‐protocol set. PWT: peak walking time.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allegra 2008 | This study is not a randomized controlled trial |
| Barker 2001 | It is unclear whether this is a double‐blind study, as it is unclear whether there was blinding on the investigators' side. Also failed on risk of bias assessment: incomplete outcome data (high risk: no P values for walking results), selective reporting (high risk: no further reporting on ABI; walking distance reported as coefficient of variation, which was not pre‐specified), and other bias (high risk: very small population (6 participants)) |
| Brevetti 1984 | Intervention in this study (L‐carnitine) is not the intervention of this review; abstract only |
| Brevetti 1988 | Intervention in this study (L‐carnitine) is not the intervention of this review |
| Brevetti 1989 | Intervention of this study (L‐carnitine) is not the intervention of this review |
| Brevetti 1996 | Outcome parameter is not a primary or secondary outcome in this review (metabolic blood markers) |
| Goldenberg 2006 | Intervention of this study (L‐carnitine) is not the intervention of this review |
| JPRN‐UMIN000016267 | This study is not a randomized controlled trial |
| Loffredo 2006 | Failed on risk of bias assessment: incomplete data (high risk: no P values comparing PLC and placebo), selective reporting (unclear: focus in this study report is on the main trial, not on this substudy), and other bias (unclear risk: very small number of participants (10), short follow‐up/treatment (7 days iv treatment), cross‐over study: 3 days placebo ‐ 3 days PLC) |
| Loffredo 2013 | Outcome parameter is not a primary or secondary outcome in this review (flow‐mediated dilation) |
| Ragozzino 2004 | This study is not a randomized controlled trial |
| Riccioni 2008 | This study is not double‐blind. Also failed risk of bias assessment: incomplete outcome data (high risk: does not report standard deviations), selection bias (no reporting of random sequence generation, no reporting of allocation concealment); blinding (high risk: no blinding), PLC monotherapy regimen alone or in association with pulsed muscular compression compared to physical therapy by itself: 3 arms: (1) infusional PLC therapy at a dosage of 4 fl (total: 1200 mg PLC) in 250 cc of physiological solution 5 days a week for 4 weeks; (2) treated with PLC in association with pulsed muscular compression therapy by Vascupump (5 sessions a week for 4 weeks); and (3) submitted only to Vascupump |
| Strano 2002 | This study is not double‐blind. Also failed risk of bias assessment: incomplete outcome data (high risk: no absolute numbers given, only mean differences, without standard deviations; figures do not match results for the mean difference; follow‐up ABI values not given) and selective reporting (high risk: major difference between ITT and PP populations (114 vs 68), indicating that results are valuable only for a select group of highly motivated participants) |
| Taylor 1996 | This study is not a randomized controlled trial |
ABI: ankle brachial index. ITT: intention‐to‐treat. iv: intravenous. PLC: propionyl‐L(evo)‐carnitine. PP: per‐protocol.
Differences between protocol and review
High risk of bias was used as an exclusion criterion; however, this is not described in the original protocol. A careful analysis of this bias estimation was performed. Given the relatively small number of participants in studies on this topic, we decided to exclude studies with additional and considerable risk of bias to minimize the overall bias in this review.
The planned sensitivity analysis 'excluding or including trials considered as debatable' was clarified as 'excluding trials that contributed large weight to the analyses'.
Contributions of authors
VK: search and selection of the literature; quality assessment; analysis and interpretation of results; writing of the review. RVS: quality assessment, interpretation of results. LC: writing of the protocol. DDB: comments on the full review. LVB: interpretation of results, comments on the full review. TDB: writing of the protocol; search and selection of the literature; quality assessment; analysis and interpretation of results; writing of the review.
Sources of support
Internal sources
-
Ghent University, Belgium
Allocation of time to work on review.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
VK: none known. RVS: none known. LC: none known. DDB: none known. LVB: received fees from the Flemish University of Brussels for expert evaluation of clinical trial conduct. He was invited to participate in the 12th INTERNATIONAL WORKSHOP, STRUCTURE AND FUNCTION OF THE VASCULAR SYSTEM in Paris in 2020, for which his travel and accommodation expenses have been reimbursed by Servier. TDB: none known.
New
References
References to studies included in this review
Andreozzi 2008 {published data only}
- Andreozzi GM, Leone A, Laudani R, Martini R, Deinite G, Cataldi V. Levo-propionyl-carnitine improves the effectiveness of supervised physical training on the absolute claudication distance in patients with intermittent claudication. Angiology 2008;59(1):84-9. [DOI] [PubMed] [Google Scholar]
Brevetti 1992 {published data only}
- Brevetti G, Perna S, Sabba C, Rossini A, Scotto di Uccio V, Berardi E, et al. Superiority of L-propionylcarnitine vs L-carnitine in improving walking capacity in patients with peripheral vascular disease: an acute, intravenous, double-blind, cross-over study. European Heart Journal 1992;13(2):251-5. [DOI] [PubMed] [Google Scholar]
Brevetti 1995 {published data only}
- Brevetti G, Perna S, Sabba C, Martone VD, Condorelli M. Propionyl-L-carnitine in intermittent claudication: double-blind, placebo-controlled, dose titration, multicenter study. Journal of American College of Cardiology 1995;26(6):1411-6. [DOI] [PubMed] [Google Scholar]
- Brevetti G, Perna S, Sabba C, Martone VD, Di Iorio A, Barletta G. Effect of propionyl-L-carnitine on quality of life in intermittent claudication. American Journal of Cardiology 1997;79(6):777-80. [DOI] [PubMed] [Google Scholar]
Brevetti 1999 {published data only}
- Brevetti G, Diehm C, Lambert D. European multicenter study on propionyl-l-carnitine in intermittent claudication. Journal of American College of Cardiology 1999;34(5):1618-24. [DOI] [PubMed] [Google Scholar]
Coto 1992 {published data only}
- Coto V, D'Alessandro L, Grattarola G, Imparato L, Lingetti M, Mancini M, et al. Evaluation of the therapeutic efficacy and tolerability of levocarnitine propionyl in the treatment of chronic obstructive arteriopathies of the lower extremities: a multicentre controlled study vs. placebo. Drugs under Experimental and Clinical Research 1992;18(1):29-36. [PubMed] [Google Scholar]
Dal Lago 1999 {published data only}
- Dal Lago A, De Martini D, Flore R, Gaetani E, Gasbarrini A, Gerardino L, et al. Effects of propionyl-L-carnitine on peripheral arterial obliterative disease of the lower limbs: a double-blind clinical trial. Drugs under Experimental and Clinical Research 1999;25(1):29-36. [PubMed] [Google Scholar]
Greco 1992 {published data only}
- Greco AV, Mingrone G, Bianchi M, Ghirlanda G. Effect of propionyl-L-carnitine in the treatment of diabetic angiopathy: controlled double-blind trial versus placebo. Drugs under Experimental and Clinical Research 1992;18(2):69-80. [PubMed] [Google Scholar]
Hiatt 2001 {published data only}
- Hiatt WR, Regensteiner JG, Creager MA, Hirsch AT, Cooke JP, Olin JW, et al. Propionyl-L-carnitine improves exercise performance and functional status in patients with claudication. American Journal of Medicine 2001;110(8):616-22. [DOI] [PubMed] [Google Scholar]
Hiatt 2011 {published data only}
- Hiatt WR, Creager MA, Amato A, Brass EP. Effect of propionyl-L-carnitine on a background of monitored exercise in patients with claudication secondary to peripheral arterial disease. Journal Cardiopulmonary Rehabilitation Prevention 2011;31(2):125-32. [NCT00399919] [DOI] [PubMed] [Google Scholar]
Luo 2013 {published data only}
- Luo T, Li J, Li L, Yang B, Liu C, Zheng Q, et al. A study on the efficacy and safety assessment of propionyl-L-carnitine tablets in treatment of intermittent claudication. Thrombosis Research 2013;132(4):427-32. [DOI] [PubMed] [Google Scholar]
- NCT00809497. A clinical study on efficacy & safety profile of propionyl-L-carnitine tablets for peripheral arterial diseases. clinicaltrialsgov/show/NCT00809497 (first received December 17, 2008).
Signorelli 2006a {published data only}
- Signorelli SS, Neri S, Di Pino L, Marchese G, Ferrante M, Oliveri CG, et al. Effect of PLC on functional parameters and oxidative profile in type 2 diabetes-associated PAD. Diabetes Research and Clinical Practice 2006;72(3):231-7. [DOI] [PubMed] [Google Scholar]
Signorelli 2006b {published data only}
- Signorelli SS, Fatuzzo P, Rapisarda F, Neri S, Ferrante M, Oliveri Conti G, et al. A randomised, controlled clinical trial evaluating changes in therapeutic efficacy and oxidative parameters after treatment with propionyl-L-carnitine in patients with peripheral arterial disease requiring hemodialysis. Drugs and Aging 2006;23(3):263-70. [DOI] [PubMed] [Google Scholar]
- Signorelli SS, Fatuzzo P, Rapisarda F, Neri S, Ferrante M, Oliveri Conti G, et al. Propionyl-L-carnitine therapy: effects on endothelin-1 and homocysteine levels in patients with peripheral arterial disease and end-stage renal disease. Kidney & Blood Pressure Research 2006;29(2):100-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allegra 2008 {published data only}
- Allegra C, Antignani PL, Schachter I, Koverech A, Messano M, Virmani A. Propionyl-L-carnitine in Leriche-Fontaine stage II peripheral arterial obstructive disease. Annals of Vascular Surgery 2008;22(4):552-8. [DOI] [PubMed] [Google Scholar]
Barker 2001 {published data only}
- Barker GA, Green S, Askew CD, Green AA, Walter PJ. Effect of propionyl-L-carnitine on exercise performance in peripheral arterial disease. Medicine and Science in Sports and Exercise 2001;33:1415-22. [DOI] [PubMed] [Google Scholar]
Brevetti 1984 {published data only}
- Brevetti G, Chiariello M, Policicchio A, Rossini A, Cavallaro C, Condorelli M. L-carnitine increases walking distance in patients with peripheral arterial disease (Abstract). European Heart Journal 1984;5(Suppl 1):138. [Google Scholar]
Brevetti 1988 {published data only}
- Brevetti G, Chiarello M, Ferulano G, Policicchio A, Nevola E, Rossini A, et al. Increases in walking distance in patients with peripheral vascular disease treated with L-carnitine: a double-blind, cross-over study. Circulation 1988;77(4):767-73. [DOI] [PubMed] [Google Scholar]
Brevetti 1989 {published data only}
- Brevetti G, Attisano T, Perna S, Rossini A, Policicchio A, Corsi M. Effects of L-carnitine on the reactive hyperemia in patients affected by peripheral vascular disease: a double-blind, cross-over study. Angiology 1989;40:857-62. [DOI] [PubMed] [Google Scholar]
Brevetti 1996 {published data only}
- Brevetti G, di Lisa F, Perna S, Menabó R, Barbato R, Martone VD, et al. Carnitine-related alternations in patients with intermittent claudication: indication for a focused carnitine therapy. Circulation 1996;93(9):1685-9. [DOI] [PubMed] [Google Scholar]
Goldenberg 2006 {published data only}
- Goldenberg NA, Krantz MJ, Hiatt WR. L-carnitine plus cilostazol verus cilostazol alone for the treatment of claudication in patients with peripheral arterial disease: a multicenter, randomized, double-blind, placebo-controlled trial. Vascular Medicine 2012;17(3):145-54. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000016267 {published data only}
- JPRN-UMIN000016267. The effect of carnitine for intermittent claudication of peripheral arterial disease patient with sarcopenia and carnitine deficiency. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000016267 (first received January 26, 2015).
Loffredo 2006 {published data only}
- Loffredo L, Pignatelli P, Cangemi R, Andreozzi P, Panico MA, Meloni V, et al. Imbalance between nitric oxide generation and oxidative stress in patients with peripheral arterial disease: effect on antioxidant treatment. Journal of Vascular Surgery 2006;44(3):525-30. [DOI] [PubMed] [Google Scholar]
Loffredo 2013 {published data only}
- Loffredo L, Carnevale R, Cangemi R, Angelico F, Augelletti T, Di SS, et al. NOX2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. International Journal of Cardiology 2013;165(1):184-92. [DOI] [PubMed] [Google Scholar]
Ragozzino 2004 {published data only}
- Ragozzino G, Mattera E, Madrid E, Salomone P, Fasano C, Gioia F, et al. Effects of propionyl-carnitine in patients with type 2 diabetes and peripheral vascular disease: results of a pilot trial. Drugs in Research and Development 2004;5(4):185-90. [DOI] [PubMed] [Google Scholar]
Riccioni 2008 {published data only}
- Riccioni C, Sarcinella R, Palermo G, Izzo A, Liguori M, Koverech A, et al. Evaluation of the efficacy of propionyl-L-carnitine versus pulsed muscular compressions in diabetic and non-diabetic patients affected by obliterating arteriopathy Leriche stage II. International Angiology 2008;27(3):253-9. [PubMed] [Google Scholar]
Strano 2002 {published data only}
- Strano A, Italian Propionyl-L-Carnitine Working Group. Propionyl-L-carnitine versus pentoxifylline: improvement in walking capacity in patients with intermittent claudication. Clinical Drug Investigation 2002;22(Suppl 1):1-6. [DOI] [PubMed] [Google Scholar]
Taylor 1996 {published data only}
- Taylor DJ, Amato A, Hands LJ, Kemp GJ, Ramaswami G, Nicolaides A, et al, MRC Biochemical and Clinical Magnetic Resonance Unit, Oxford Radcliffe Hospital Trust, UK. Changes in energy metabolism of calf muscle in patients with intermittent claudication assessed by 31P magnetic resonance spectroscopy: a phase II open study. Vascular Medicine (London, England) 1996;1(4):241-5. [DOI] [PubMed] [Google Scholar]
Additional references
Andreozzi 2009
- Andreozzi GM. Propionyl-l-carnitine: intermittent claudication and peripheral arterial disease. Expert Opinion on Pharmacotherapy 2009;10(16):2697-707. [DOI] [PubMed] [Google Scholar]
Brass 1998
- Brass EP, Hiatt WR. The role of carnitine and carnitine supplementation during exercise in man and in individuals with special needs. Journal of the American College of Nutrition 1998;17(3):207-15. [DOI] [PubMed] [Google Scholar]
Brass 2013
- Brass EP, Koster D, Hiatt WR, Amato A. A systematic review and meta-analysis of propionyl-l-carnitine effects on exercise performance in patients with claudication. Vascular Medicine 2013;18(1):3-12. [DOI] [PubMed] [Google Scholar]
Brevetti 1997
- Brevetti G, Fanin M, De Amicis V, Carrozzo R, Di Lello F, Martone VD, et al. Changes in skeletal muscle histology and metabolism in patients undergoing exercise deconditioning: effect of propionyl-L-carnitine. Muscle and Nerve 1997;20(9):1115-20. [DOI] [PubMed] [Google Scholar]
Brown 2021
- Brown T, Forster RB, Cleanthis M, Mikhailidis DP, Stansby G, Stewart M. Cilostazol for intermittent claudication. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD003748. [DOI: 10.1002/14651858.CD003748.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Backer 2010
- De Backer TLM, Vander Stichele R, De Buyzere M, De Backer G, Van Bortel L. Silence of the limbs: pharmacological symptomatic treatment of intermittent claudication. Current Vascular Pharmacology 2010;8(3):383-7. [DOI] [PubMed] [Google Scholar]
De Backer 2012
- Backer TLM, Vander Stichele R, Lehert P, Van Bortel L. Naftidrofuryl for intermittent claudication. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD001368. [DOI: 10.1002/14651858.CD001368.pub4] [DOI] [PubMed] [Google Scholar]
Deckert 1997
- Deckert J. Propionyl-L-carnitine for intermittent claudication. Journal of Family Practice 1997;44(6):533-4. [PubMed]
Delaney 2013
- Delaney CL, Spark JI, Thomas J, Wong YT, Chan LT, Miller MD. A systematic review to evaluate the effectiveness of carnitine supplementation in improving walking performance among individuals with intermittent claudication. Atherosclerosis 2013;229(1):1-9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2003
- Evans AM, Fornasini G. Pharmacokinetics of L-carnitine. Clinical Pharmacokinetics 2003;42(11):941-67. [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripherern Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;5/6:499-533. [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FG, Housley E, Cawood EH, MacIntyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International Journal of Epidemiology 1991;20(2):384-92. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed April 13, 2018. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ. GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed.) 2008;336(7651):995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Hiatt 1995
- Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation 1995;91(5):1472-9. [DOI] [PubMed] [Google Scholar]
Hiatt 2004
- Hiatt WR. Carnitine and peripheral arterial disease. Annals of the New York Academy of Sciences 2004;1033:92-8. [DOI] [PubMed] [Google Scholar]
Hiatt 2008
- Hiatt WR, Goldstone J, Smith S, McDermott M, Moneta G, Oka R, Newman A, Pearce WH. Atherosclerotic Peripheral Vascular Disease Symposium II. Nomenclature for vascular diseases. Circulation 2008;118(25):2826–9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6. Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Meijer 2002
- Meijer WT, Cost B, Bernsen RM, Hoes AW. Incidence and management of intermittent claudication in primary care in The Netherlands. Scandinavian Journal of Primary Health Care 2002;20(1):33-4. [DOI] [PubMed] [Google Scholar]
Milio 2009
- Milio G, Novo G, Genova C, Almasio P, Novo S, Pinto A. Pharmacological treatment of patients with chronic critical limb ischemia: L-propionyl-carnitine enhances the short-term effects of PGE-1. Cardiovascular Drugs and Therapy 2009;23(4):301-6. [DOI] [PubMed] [Google Scholar]
Moher 2000
- Moher D, Pham B, Ausejo M, Saenz A, Hood S, Barber GG. Pharmacological management of intermittent claudication: a meta-analysis of randomized trials. Drugs 2000;59(5):1057-70. [DOI] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). European Journal of Vascular and Endovascular Surgery 2007;33 (Suppl 1):1-75. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Sigvant 2007
- Sigvant B, Wiber-Hedman K, Bergqvist D, Rolandsson O, Anderson B, Persson E, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. Journal of Vascular Surgery 2007;45(6):1185-91. [DOI] [PubMed] [Google Scholar]
Stephens 2007
- Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. Journal of Physiology 2007;581(Pt 2):431-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
de Backer 2012
- Backer TLM, Campens L, Vander Stichele R, Van Bortel L, De Bacquer D. Propionyl‐L‐carnitine for intermittent claudication. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD010117. [DOI: 10.1002/14651858.CD010117] [DOI] [PMC free article] [PubMed] [Google Scholar]


