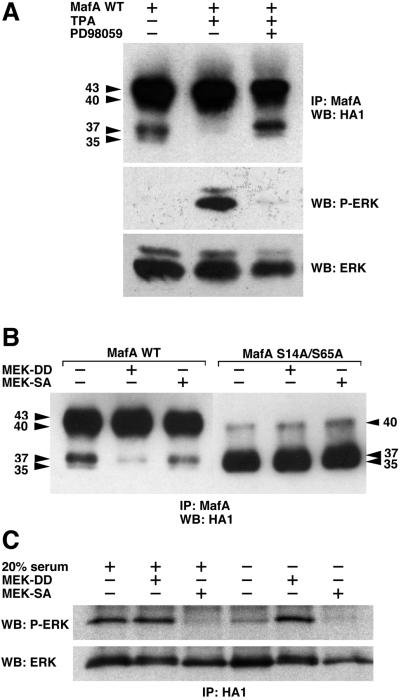
FIG. 4.
MafA serines 14 and 65 can be phosphorylated in vivo in response to activation of the MEK/ERK pathway. (A) HeLa cells were transiently transfected with pcDNA3-derived vectors encoding WT MafA and either serum-starved (−), treated with TPA (TPA), or treated with TPA in the presence of PD98059 (PD98059). Cellular extracts were immunoprecipitated with MafA-directed antiserum and analyzed by Western blotting with anti-HA1 antibody. The status of ERK activation was controlled as follows: protein extracts from identical cultures were analyzed by Western blotting with anti-phospho-ERK antibody, stripping of the membranes, and reprobing with anti-ERK antibody. (B) HeLa cells were transiently transfected with pcDNA3-derived vectors encoding either WT MafA (WT) or S14A/S65A MafA (S14A/S65A) and with expression vectors for MEK1S218D/S222D (MEK-DD), MEK1S222A (MEK-SA), or empty vector (−). Cell lysates were treated as described for panel A. (C) Control of the constitutively active and dominant negative property of the respective S218D/S222D and S222A MEK1 mutants. Cultures of HeLa cells were transfected as described for panel B, with the addition of an HA-ERK2 expression plasmid in the transfection mix. The status of ERK activation in the presence of either MEK-DD or MEK-SA was analyzed under two experimental conditions. In the first three lanes (left side), serum-starved cultures were stimulated for 10 min by addition of medium containing 20% FBS. In the last three lanes (right side), cells were maintained in culture without any additional stimulation, as in panel B. Cellular extracts were immunoprecipitated with HA1-directed antibody and analyzed by Western blotting with anti-phospho-ERK antibody, stripping of the membranes, and reprobing with anti-ERK antibody.