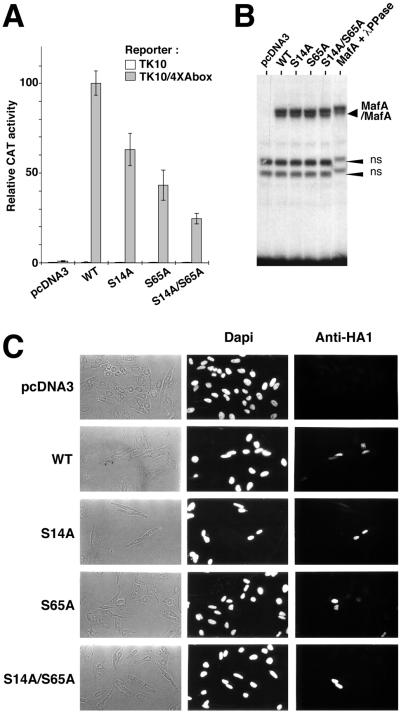
FIG. 5.
Serines 14 and 65 are required for MafA transcriptional activity. (A) Quail NR cells infected with tsNY68 virus were transiently transfected with the TK10/4×Abox reporter or empty TK10 plasmid and pcDNA3-derived vectors either empty or encoding WT, S14A, S65A, or S14A/S65A versions of MafA. CAT activity was measured at 41°C and expressed as a relative value with respect to the TK10/4×Abox and pcDNA3-WT transfection. Results represent mean values from four experiments. Expression levels for WT and mutant MafA proteins were controlled by Western blotting in each experiment (data not shown). (B) Serine 14 and serine 65 residues are not required for MafA DNA binding. WT or mutated MafA proteins encoded by pcDNA3-derived plasmids were obtained by in vitro transcription and translation in reticulocyte lysates and subjected to gel-shift analysis with a 32P-labeled A box probe. MafA + λPPase indicates that the WT MafA-containing lysate was incubated with λ phosphatase prior to DNA binding. MafA/MafA indicates MafA homodimers bound to the probe. ns, nonspecific bands. (C) Integrity of serine 14 and serine 65 residues is not required for MafA nuclear localization. HeLa cells were transiently transfected with pcDNA3-derived vectors encoding WT, S14A, S65A, or S14A/S65A forms of MafA and analyzed by immunofluorescence with anti-HA1 antibody.