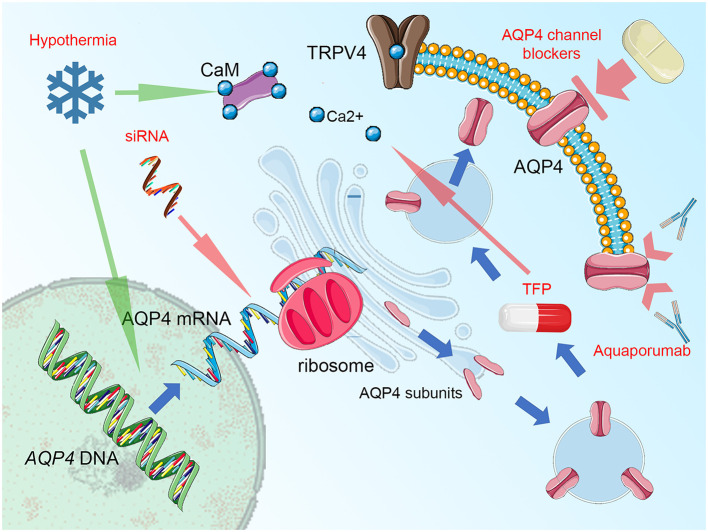
Figure 1.
Summary figure, demonstrating aquaporin-4 (AQP4) cellular trafficking as a possible target for treatment. Blue arrows represent the process of AQP4 production and relocation, the groups of potential therapeutics are labeled by red text and their impact is marked by green (enhancing) or red arrows (blocking activity). AQP4 expression (transcription of the AQP4 gene and translation of AQP4 mRNA with ribosomal production of AQP single subunits may be disturbed by small interfering RNA (siRNA), attaching selectively to AQP4 mRNA domains and preventing the translational readout. The single subunits of AQP4 are organized into orthogonal arrays of particles (OAPs) and as tetramers are transferred by endosomal vesicles to the proximity of cell membrane (predominantly in astrocytic endfoot area). Here, the AQP4 translocation to the cell surface takes place. This process relies on the activity of vanilloid-receptor-related subfamily 4 calcium channel (TRPV4) and calmodulin (CaM), directly binding to the AQP4 particles. Importantly, blocking CaM activity by trifluoperazine (TFP) was efficient against AQP4 relocation and the formation of cytotoxic brain edema. Notably, hypothermia exerts opposite action enhancing AQP4 surface exposition and this effect may be counteracted by TRPV4 inhibitors, Ca2+ chelating compounds, or CaM blockers. This effect is more relevant than the impact of hypothermia on AQP4 expression, with increased transcription reported by some, but not all relevant studies. The AQP4 channel, while integrated into astrocytic surface membrane, may be simply blocked by a number of compounds, including acetazolamide, topiramate, lamotrigine, zonisamide, acetylsulfanilamide, phenytoin, bumetanide, furosemide, tetraethylammonium, and IMD0354 as well as by heavy metal derivates or—more selectively—by TGN-020. In conditions of autoimmune response that is driven against AQP4 channels, as seen in neuromyelitis optica (NMO), blocking of antigen epitopes by monoclonal antibodies (aquaporumab), has been demonstrated as an effective NMO treatment, at least in experimental conditions. Figure created with the use of Servier Medical Art images/content of smart.servier.com in compliance with the terms of the Creative Commons Attribution 3.0 Unported Licence.