Figure 1.
Identification of Senp5S isoform
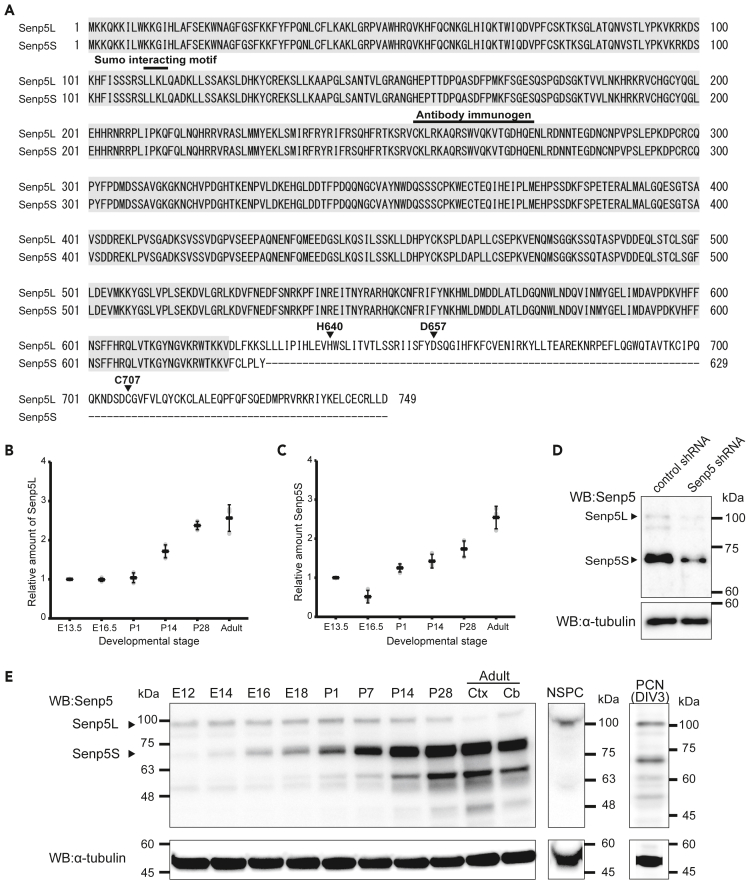
(A) The deduced amino acid sequences of mouse Senp5L and Senp5S. Identical amino acids are highlighted. Gaps in the alignment are indicated by dashes. Arrowhead denotes the catalytic center Cys-707 necessary for peptidase activity. His-640, Asp-657, and Cys-707 constitute the catalytic triad for cysteine protease. The N-terminal LLKL sequence (110–113) is the SUMO-interacting motif. Only Senp5S lacks the C-terminal catalytic center, although both isoforms have the N-terminal SUMO-interacting motif.
(B and C) Developmental changes in Senp5L (B) and Senp5S (C) mRNA expression in the mouse cerebral cortex by qPCR analysis. Gray dots represent three independent experiments normalized to the corresponding β-actin mRNA. Mean ± SD are also shown.
(D) PCNs prepared from mouse embryonic brains were electroporated with Senp5 shRNA or non-targeting control shRNA and cultured for 3 div. Immunoblot with anti-Senp5 or α-tubulin antibody demonstrated the suppression of endogenous protein expression of Senp5L and Senp5S.
(E) Developmental changes in Senp5L/5S protein expression in the mouse brain. Total protein extracts were prepared from the whole brains (E12–P28), adult cerebral cortex (ctx), or adult cerebellum (cb) and subjected to immunoblotting with anti-Senp5 antibody (left panel). Protein lysate from primary cultured neural stem/precursor cells (NSPC) or primary cultured cortical neurons (PCN) at 3 div was also analyzed by immunoblot (middle and right panels). The blots were reprobed with anti-α-tubulin antibody (bottom panels) to examine protein loading quantitatively.