Abstract
Background
Doxorubicin (Dox) is a potent chemotherapeutic agent, but its usage is limited by dose-dependent cardiotoxicity. Intracellular calcium dysregulation has been reported to be involved in doxorubicin-induced cardiomyopathy (DICM). The cardioprotective role of RyR stabilizer dantrolene (Dan) on the calcium dynamics of DICM has not yet been explored.
Objective
To evaluate the effects of dantrolene on intracellular calcium dysregulation and cardiac contractile function in a DICM model.
Methods
Adult male C57BL/6 mice were randomized into 4 groups: (1) Control, (2) Dox Only, (3) Dan Only, and (4) Dan + Dox. Fractional shortening (FS) and left ventricular ejection fraction (LVEF) were assessed by echocardiography. In addition, mice were sacrificed 2 weeks after doxorubicin injection for optical mapping of the heart in a Langendorff setup.
Results
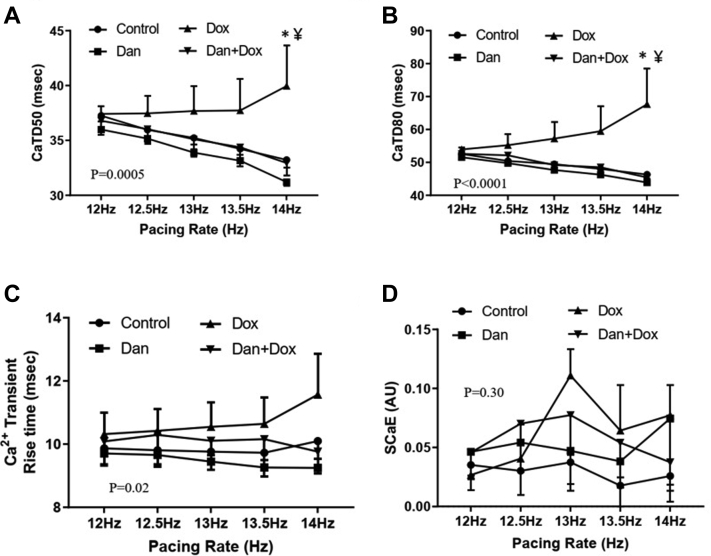
Treatment with Dox was associated with a reduction in both FS and LVEF at 2 weeks (P < .0001) and 4 weeks (P < .006). Dox treatment was also associated with prolongation of calcium transient durations CaTD50 (P = .0005) and CaTD80 (P < .0001) and reduction of calcium amplitude alternans ratio (P < .0001). Concomitant treatment with Dan prevented the Dox-induced decline in FS and LVEF (P < .002 at both 2 and 4 weeks). Dan also prevented Dox-induced prolongation of CaTD50 and CaTD80 and improved the CaT alternans ratio (P < .0001). Finally, calcium transient rise time was increased in the doxorubicin-treated group, indicating RyR2 dyssynchrony, and dantrolene prevented this prolongation (P = .02).
Conclusion
Dantrolene prevents cardiac contractile dysfunction following doxorubicin treatment by mitigating dysregulation of calcium dynamics.
Keywords: Cardiomyopathy, Cardiac function, Doxorubicin, Dantrolene, Myocardial calcium transients
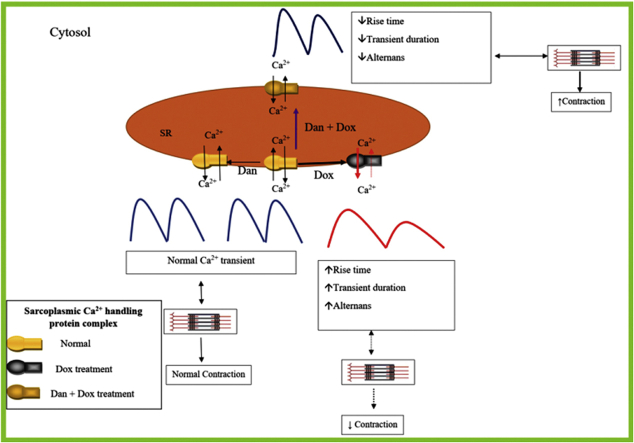
Graphical abstract
Key Findings.
-
▪
Treatment with single-dose doxorubicin was associated with a persistent decline in left ventricular fractional shortening (FS) and ejection fraction in a murine model.
-
▪
Doxorubicin treatment was also associated with prolongation of calcium transient (CaT) rise time and calcium transient duration (CaTD, both CaTD50 and CaTD80), as well as higher calcium transient alternans.
-
▪
Concurrent administration of dantrolene prevented doxorubicin-induced prolongation of CaT rise time and CaTD, as well as reduction of calcium transient alternans.
-
▪
Correction of doxorubicin-induced calcium dysfunction by dantrolene was associated with mitigation of reduction of FS and left ventricular ejection fraction.
Introduction
Doxorubicin (Dox) is an anthracycline drug that has been used as a chemotherapeutic agent since the 1960s.1 Dox works by inducing cellular toxicity and is used in the treatment of many cancers, including solid tumors and hematological malignancies.2 However, the clinical uses of this drug are limited owing to short-term and long-term cardiotoxic effects.2 Systolic dysfunction and loss of cardiomyocytes are hallmarks of doxorubicin-induced cardiomyopathy (DICM).2 Calcium dysregulation has emerged as one of the major pathophysiological features of DICM,3,4 and elevated cytosolic calcium level has been found to precede morphologic and clinical cardiomyopathy.5 Calcium dysregulation in DICM is associated with cardiac dysfunction and cardiomyocyte loss.6 Calcium dysregulation is also linked to reactive oxygen species (ROS) generation.6,7 Increased ROS production and subsequent interaction of Dox with sarcoplasmic calcium-handling proteins are believed to result in the abnormal calcium dynamics associated with DICM.8
Studies by our group,9,10 as well as by others, have also demonstrated an association between Dox treatment and abnormal calcium dynamics.4 Apart from contractile dysfunction and arrhythmia, calcium dysregulation also plays a crucial role in mitochondrial dysfunction, hypertrophic remodeling, and myocyte loss in cardiomyopathies and heart failure.11 Thus, targeting the normalization of aberrant calcium dynamics as a therapeutic paradigm could protect DICM.12 The current clinical approach for DICM includes regular screening for early detection of left ventricle (LV) dysfunction and standard heart failure treatment in the presence of ventricular dysfunction. To date, effective strategies to treat and prevent Dox-induced cardiomyopathy are limited.13 To prevent chemotherapy-induced cardiomyopathy, concurrent administration of carvedilol has been presented as a possible therapeutic regimen, but the effectiveness of such an approach remains uncertain.14 Other strategies, such as treatments with ROS scavengers15 or antioxidants,12,16 have not been able to protect against DICM. Dexrazoxane, an iron chelator, has been approved to treat DICM, but it weakens the antitumor effect of the chemotherapy, and the emergence of secondary malignancies is a concern.17 Alternatively, dantrolene (Dan), a ryanodine receptor (RyR) stabilizer, has been shown to improve sarcoplasmic calcium release as well as cardiac function in models of both acute and chronic cardiomyopathy.18, 19, 20, 21 Previously, we have shown that Dan infusion during cardiopulmonary resuscitation improved cardiac hemodynamics by normalizing cardiac calcium dynamics.22 Thus, in the present study, we sought to investigate the role of dantrolene-mediated calcium regulation in the prevention of DICM using a murine model.
Materials and methods
Experiment protocol
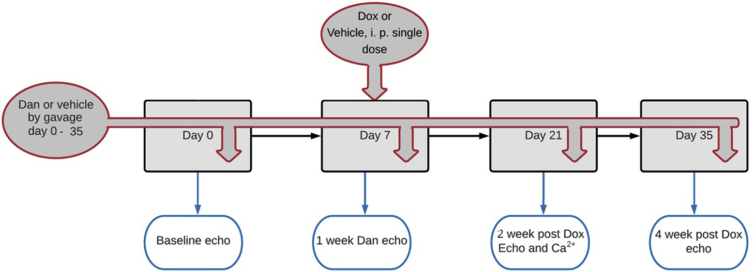
The experiments were performed in University Health Network. All animal usage was in accordance with institutional animal care guidelines and was approved by the Animal Care Committee of the University Health Network (Canadian Council in Animal Care). Male C57BL/6J mice (Jackson Labs, Bar Harbor, ME) aged 10–12 weeks old were used. Mice were acclimated at the housing facility for at least 1 week before the experimental protocol. Treatment with dantrolene (D9175; Sigma-Aldrich, Oakville, ON, Canada) was started 7 days before Dox treatment. Mice were treated with a single intraperitoneal (i.p.) dose of doxorubicin (10 mg/kg, D1515; Sigma-Aldrich) or distilled water (DW) on day 7 of dantrolene treatment. Our previous study has demonstrated that a 10 mg/kg dose of doxorubicin is successful in inducing sustained cardiomyopathy.9 Echocardiography was subsequently conducted every week to monitor changes in cardiac function. In our preliminary experiments on dantrolene, daily doses of 1 mg/kg, 2 mg/kg, and 5 mg/kg were shown to have no significant effects on fractional shortening (FS) reduction as compared to doxorubicin. However, dantrolene at a daily dose of 10 mg/kg was shown to prevent the reduction of FS following doxorubicin treatment; as such, this dose of dantrolene was used in all subsequent treatments. Two weeks after Dox administration, the hearts of the mice were harvested and perfused in a Langendorff setup. Calcium mapping was then performed in the presence of calcium-sensitive fluorescent dye. The experimental protocol is outlined in Figure 1.
Figure 1.
A schematic representation of the experimental protocol.
Experimental groups
The mice were randomized into 4 experimental groups: group 1 (Control) received oral and i.p. DW; group 2 (Dan only) received dantrolene by oral gavage with i.p. DW simulating doxorubicin injection; group 3 (Dox only) received doxorubicin i.p. on day 7 with daily oral gavage of DW, and group 4 (Dan + Dox) received oral dantrolene 10 mg/kg daily and doxorubicin i.p. on day 7 (Figure 1).
Echocardiography
Echocardiography was performed weekly on anesthetized mice (2% isoflurane) in all experimental groups during the 35-day protocol, as shown in Figure 1. M-mode measurements of the LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) were measured using a 15 MHz linear ultrasound transducer (Vivid7; GE, Boston, MA) with the body temperature of the mice maintained at 37°C. From short-axis view, at the level of the papillary muscle, the measurement were performed in triplicate and averaged over 3–6 beats. LVEDD was measured at the maximal LV end-diastolic dimension, whereas LVESD was measured at the minimum LV systolic excursion. LV FS was used as an indicator of murine cardiac function and was calculated as:
FS = (LVEDD – LVESD) / LVEDD × 100%
LV end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were determined by the software based on LV dimension measurements and used to calculate LV ejection fraction (EF) as a secondary measure of cardiac function:
EF = (LVEDV – LVESV) / LVEDV × 100%
Measurements were reported as mean ± SEM for all groups at the prespecified time points. The primary study outcome was the prevention of a doxorubicin-induced reduction of FS as measured by echocardiography.
Calcium mapping in Langendorff setup
Two weeks after doxorubicin treatment, each mouse was deeply anesthetized with 2%–3% isoflurane and the hearts were harvested via midline thoracotomy. Each murine heart was immediately immersed into an ice-cold modified Krebs-Henseleit solution containing (in mM): NaCl (118), KCl (4.7), CaCl2 (1.3), MgSO4 (1.2), KH2PO4 (1.2), NaHCO3 (25), and glucose (5.5). The aortic stub was then cannulated to allow for retrograde perfusion of the coronary vessels with the modified Krebs-Henseleit solution. The perfusion pressure was approximately 70 mm Hg and the temperature was maintained at 37°C. Following stabilization of the explanted heart, the calcium-sensitive fluorescent dye Rhod2-AM (0.07 μM; Biotium, Fremont, CA) and mechanical uncoupler Blebbistatin (1.0 μM; Enzo Life Sciences, Farmingdale, NY) were added to the perfusion solution to allow for optical mapping of the heart. Using a xenon light source (Moritex Corporation, Saitama, Japan) and a 530 nm green filter (Semrock) Rhod2-AM fluorescence was excited and the light emission bandpass filtered at 585/40 nm. Dye fluorescence was recorded with a high-speed CMOS camera (Ultima-L, Scimedia, Costa Mesa, CA) at a sampling rate of 500 frames/second. Image size was 16 mm × 16 mm with a 100 × 100-pixel resolution. Following a pace-and-pause protocol, optical mapping of calcium signals from the ventricular epicardium was performed to assess myocardial calcium dynamics. Briefly, a Grass S88X stimulator was used to pace the Langendorff-perfused heart for 30 seconds, via 2 electrodes attached to the epicardial surface, to reach a steady state of 12–14 Hz. Fluorescence was recorded at the end of the pacing maneuver and the beginning of spontaneous beats. Optical signals recorded during the final 2 seconds of pacing and the first 2 seconds of spontaneous rhythm were analyzed. From an area on the optical maps representing the ventricle, 3 non-adjacent pixels were selected for the following feature analysis of calcium signals using custom-made MATLAB codes:
Calcium transient duration analysis
For calcium transient duration (CaTD) analysis, 0 to 50% of repolarization (CaTD50) and 0 to 80% of repolarization (CaTD80) were analyzed, as described previously.23,24
Calcium amplitude alternans ratio analysis
Ca2+ amplitude alternans refers to the beat-to-beat difference of Ca2+ signal amplitudes during cardiac stimulation. A calcium amplitude alternans ratio (CaAAR) of 1 indicates similar amplitudes, whereas a ratio of 0.5 refers to a 50% difference between large and small Ca2+ amplitudes. As described in our previous publications, this parameter was calculated as a ratio (smallest Ca2+ signals)/(largest Ca2+ signals), where the largest and smallest Ca2+ signals were each derived from either odd or even beats.24,25
Calcium transient rise time analysis
Rise time of calcium transient upstrokes (calcium transient [CaT] rise time) was calculated as the time duration for fluorescence to rise from minimum (10%) to maximum (90%) and was expressed in milliseconds.26
Diastolic/spontaneous calcium leak
The presence of spontaneous calcium elevation (SCaE) is the result of a leaky RyR2 receptor during diastole. This parameter is unitless and was measured from the Ca2+ wave and normalized to Ca2+ transient amplitude. The calcium signal fluorescence elevation between the final pacing beat and the first spontaneous beat was calculated as described previously.24
Statistical analysis
The study was powered to demonstrate the effect of dantrolene on FS. No previous study has evaluated the role of dantrolene treatment on changes in FS in a DICM model. We hypothesize that there will be no reduction in FS with concurrent administration of dantrolene with doxorubicin (Dan + Dox group). The sample size was calculated from our previous study examining the effects of doxorubicin single-dose injection (10 mg/kg).9 The calculations were based on a P value of .05 (Zα/2= 1.96 at 95% confidence interval) and an error rate of 1%. Based on previous animal studies, we expected to measure a difference in FS between the control and Dox groups of approximately 25%. The minimum sample size for comparing 2 means of continuous variables was calculated with the formula: n = (Zα/2)2 ∗σ2 /E2, where σ2 is the standard deviation (1.1) and E is the error rate (1%). This yielded a value of n = 5 for each group. Echocardiographic measurements were reported as mean ± SEM for all groups at the prespecified time points. FS; EF; calcium signals for CaTD50 and CaTD80; CaAAR; rise time; and SCaE were analyzed by 2-way ANOVA (ordinary for Ca2+ and mixed effect for FS and EF, followed by Bonferroni test). A P value <.05 is considered to be statistically significant.
Results
Effect on dantrolene on cardiac contractile function
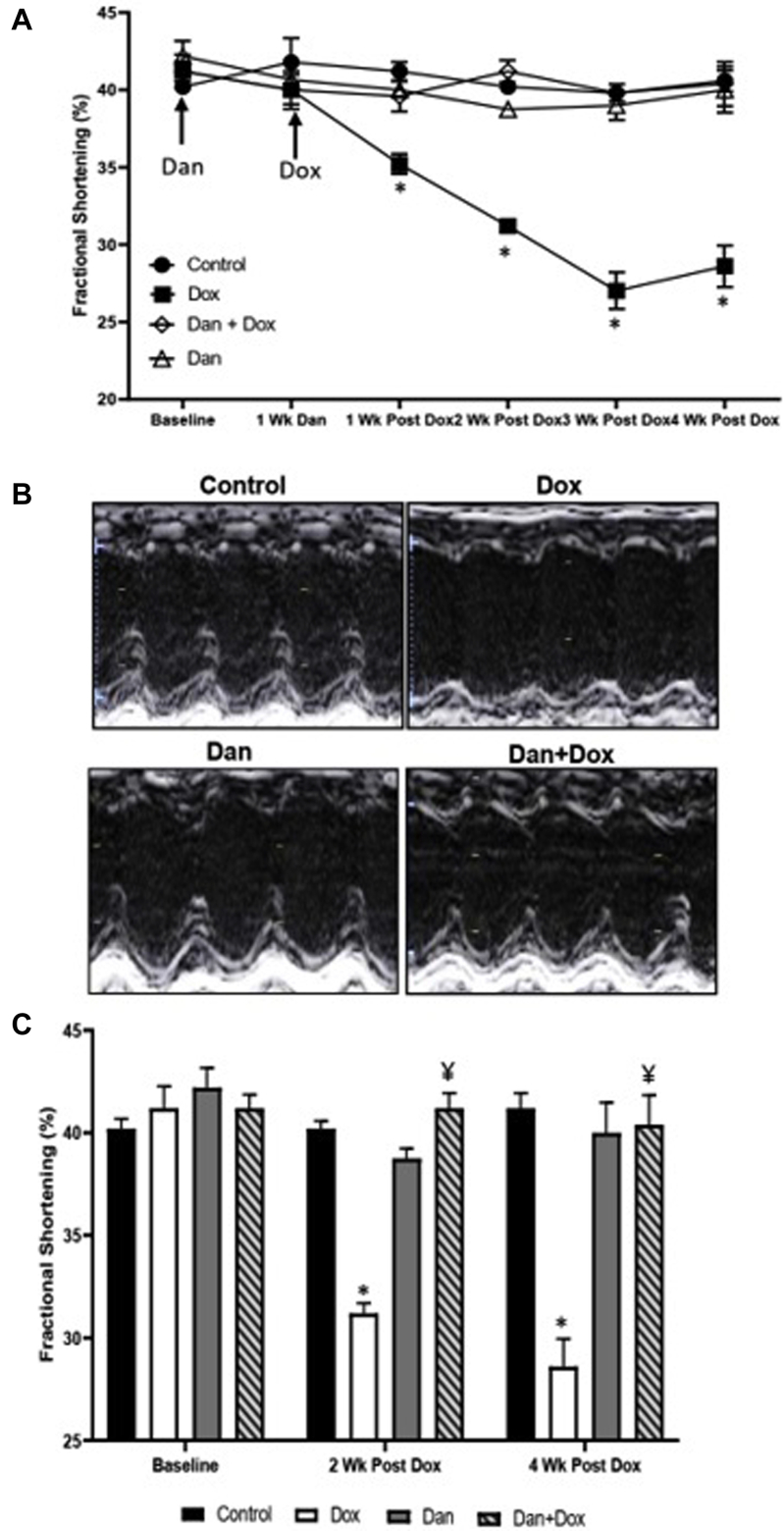
Baseline FS was comparable in all groups (Figure 2). At 2 weeks, a relative drop of 22% in FS was observed in the Dox only group compared to control. More specifically, FS was 40.20% ± 0.37%, 38.75% ± 0.48%, 31.20% ± 0.49%, and 41.20% ± 0.73% in control, Dan only, Dox only, and Dan + Dox, respectively (P < .0001, control vs Dox only and P < .0001, Dox only vs Dan + Dox; Figure 2) after 2 weeks of treatment.
Figure 2.
Temporal effects of dantrolene (Dan) on fractional shortening (FS) following doxorubicin (Dox) treatment. A: Effects of Dan 10 mg/kg daily on FS following Dox treatment. Echocardiography was performed at baseline, after day 7 of Dan treatment, and 1, 2, 3, and 4 weeks after Dox treatment. FS was significantly reduced 1 week following the Dox administration and sustained up to the observation period of 4 weeks. Treatment with Dan prevented the decline in FS after Dox treatment. Asterisk (∗) indicates significant differences at 1 week after the injection of Dox (P = .0009 control vs Dox only; P = .04 Dox vs Dan + Dox), 2 weeks after the injection of Dox (P < .0001 control vs Dox only; P < .0001 Dox vs Dan + Dox), 3 weeks after the injection of Dox (P = .0005 control vs Dox only; P = .001 Dox only vs Dan + Dox), and 4 weeks after the injection of Dox (P = .001 control vs Dox; P = .002 Dox vs Dan + Dox). N = 4–5 mice per group. B: Representative images of M-mode echocardiography at 4 weeks after Dox treatment. C: Effects of Dan at 2 and 4 weeks following Dox treatment. FS as measured by echocardiography in the 4 groups. Asterisk (∗) denotes significant difference vs controls (P < .0001 at 2 weeks and P = .001 at 4 weeks after Dox injection) and ¥ denotes significant difference vs Dox group (P < .0001 at 2 weeks and P = .002 at 4 weeks after Dox injection). N = 4–5 mice per group.
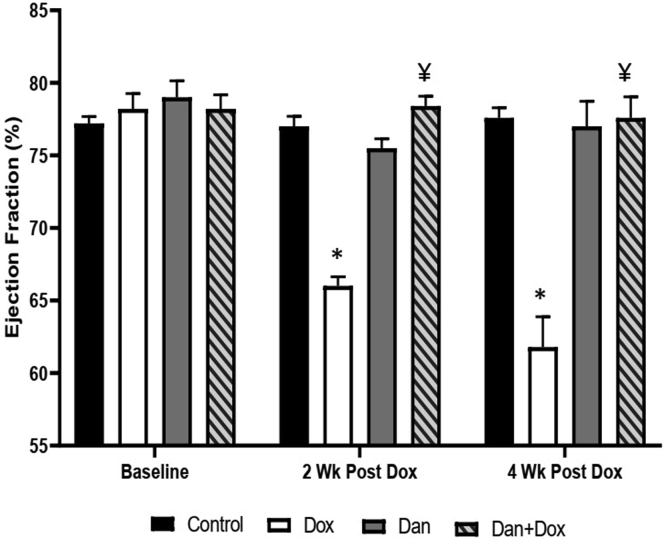
This drop in FS persisted for 4 weeks after doxorubicin treatment. At 4 weeks, the FS was 41.20% ± 0.74%, 40.00% ± 1.47%, 28.60% ± 1.36%, and 40.40% ± 1.44% in control, Dan only, Dox only, and Dan + Dox, respectively (P = .001, control vs Dox only and P = .002, Dox only vs Dan + Dox; Figure 2). A similar drop in LVEF was seen after 2 weeks (77.0% ± 0.71%, 75.5% ± 0.65%, 66.0% ± 0.63%, and 78.4% ± 0.68% in control, Dan only, Dox only, and Dan + Dox, respectively; P < .0001, control vs Dox only and P < .0001, Dox only vs Dan + Dox; Figure 3). This correlated with a relative drop of 14% in LVEF in the Dox only group, which was not seen in any of the groups that were treated with dantrolene. At 4 weeks post-doxorubicin (or vehicle), the EF was 77.6% ± 0.68%, 77.0% ± 1.73%, 61.8% ± 2.08%, and 77.6% ± 1.44% in control, Dan only, Dox only, and Dan + Dox, respectively; P = .006, control vs Dox only and P = .002, Dox only vs Dan + Dox (Figure 3).
Figure 3.
Effects of dantrolene (Dan) on ejection fraction following doxorubicin (Dox) treatment. Ejection fraction was measured by echocardiography in the 4 groups at 2 and 4 weeks following Dox treatment as described in Methods section. Asterisk (∗) denotes significant difference vs controls (P < .0001 at 2 weeks and P = .006 at 4 weeks) and ¥ denotes significant difference vs Dox only group (P < .0001 at 2 weeks and P = .002 at 4 weeks). N = 4–5 mice per group.
Effects of dantrolene on calcium transient duration
Next, we studied calcium dynamics in a Langendorff setup of isolated murine hearts. The effects of a 5-week dantrolene treatment regimen on the mitigation of Dox-induced calcium dysregulation were assessed. As compared to the control group, Dox lengthened CaTD50 (39.96 ± 3.67 ms vs 33.24 ± 0.68 ms at 14 Hz, P = .02, Figure 4A). A more pronounced prolongation was seen in CaTD80 (67.6 ± 10.88 ms vs 46.32 ± 1.17 ms at 14 Hz, P = .001, Figure 4B). The prolongation of CaTD50 (32.95 ± 1.15 ms vs 39.96 ± 3.67 at 14 Hz, P = .007, Figure 4A) and CaTD80 (45.43 ± 1.57 ms vs 67.65 ± 10.88 ms at 14 Hz, P = .0003, Figure 4B) was prevented with dantrolene treatment. When compared with controls, the Dan only group did not have a significantly different CaTD50 or CaTD80 (Figure 4A and 4B).
Figure 4.
Effects of dantrolene (Dan) on calcium transient dynamics following doxorubicin (Dox) treatment. A: Calcium transient duration 50 (CaTD50) at different pacing rates. Asterisk (∗) denotes P = .02 vs control group and ¥ denotes P = .007 vs Dan + Dox-treated group. Overall, P = .0005 by 2-way ANOVA among the 4 groups. N = 4–6 in each group. B: Calcium transient duration 80 (CaTD80) at different pacing rates. Asterisk (∗) denotes P = .001 vs controls, and ¥ denotes P = .0003 vs Dan + Dox-treated group. Overall, P < .0001 by 2-way ANOVA among the 4 groups. N = 4–6 mice per group. C: Calcium transient rise time. Rise time was calculated from the optical signal as described in the Methods section. Overall, P = .02 by 2-way ANOVA among the 4 groups. N = 4–6 in each group. D: Spontaneous calcium elevation (SCaE). SCaE was measured from the optical signal as described in Methods section. Overall P = .30 by 2-way ANOVA among the 4 groups. N = 4–6 mice per group.
Effects of dantrolene on calcium transient rise time
Dox treatment was associated with an increased rise time compared to control hearts (11.55 ± 1.30 ms in Dox only vs 10.10 ± 0.56 ms in control at 14 Hz, overall P = .02, Figure 4C). The treatment with Dan prevented the prolongation of rise time following Dox treatment (11.55 ± 1.30 ms in Dox only vs 9.76 ± 0.58 ms in Dan + Dox at 14 Hz, overall P = .02, Figure 4C). These data lend support to the mechanism of Dox-induced RyR2 dysregulation, as well as the role of dantrolene in the stabilization of RyR2 activity.
Effects of dantrolene on diastolic calcium leak
We measured the elevation of diastolic calcium amplitude to explore the effects of dantrolene on diastolic calcium leak following doxorubicin treatment. We found that there was an elevation of spontaneous diastolic calcium in the hearts of Dox-treated mice compared to control groups (P = .046); however, Dan had no effects on elevated SCaE (P = .30, Figure 4D).
Effects of dantrolene on CaAAR
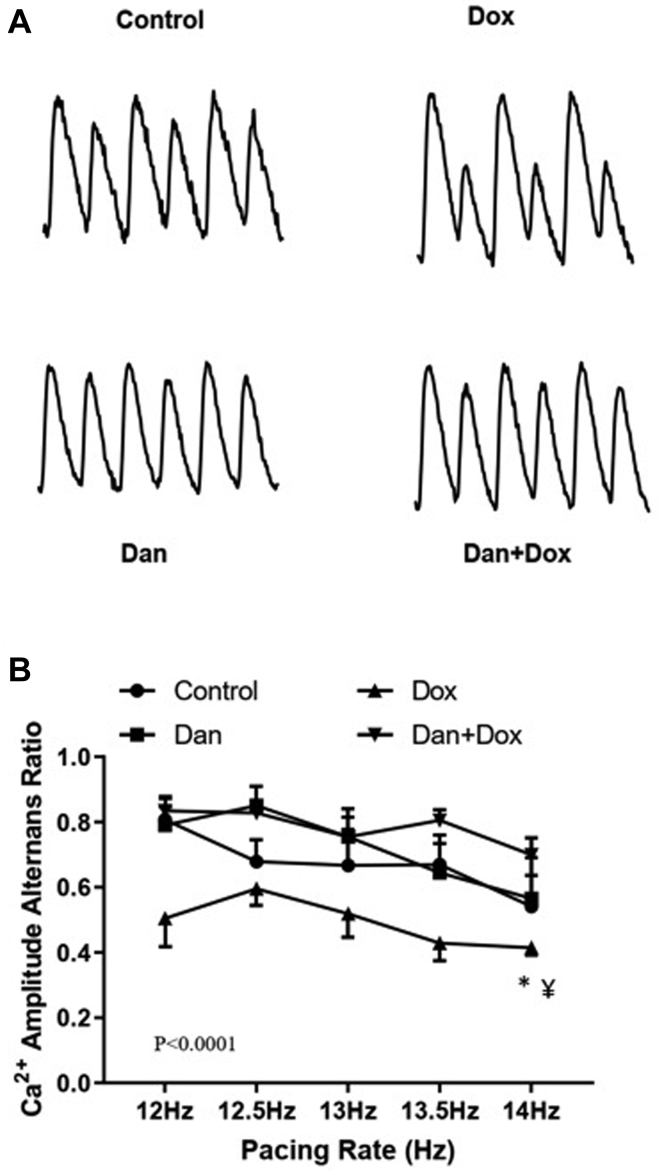
Beat-to-beat CaAAR was significantly lower in the Dox-treated group compared to the controls (0.42 ± 0.02 in Dox only group vs 0.54 ± 0.09 in the control group at 14 Hz, overall P < .0001). Dantrolene prevented the reduction of CaAAR following Dox treatment (0.42 ± 0.02 in the Dox only group vs 0.70 ± 0.05 in the Dan + Dox group at 14 Hz, P = .04, Figure 5). These data further suggest that RyR2 function is dysregulated following Dox treatment.
Figure 5.
Effects of dantrolene (Dan) on Ca2+ amplitude alternans ratios (CaAAR) following doxorubicin (Dox) treatment. CaAAR was measured from the optical signal as described in the Methods section. a: Representative fluorescence tracings showing the calcium amplitude alternans in different groups. b: Graphical presentation of CaAAR at different pacing rates. Asterisk (∗) denotes P < .0001 vs control and ¥ denotes P = .04 vs Dan + Dox-treated group. Overall, P < .0001 by 2-way ANOVA among the 4 groups. N = 4–6 mice per group.
Discussion
In this study, we investigated the cardioprotective role of dantrolene in doxorubicin-induced cardiomyopathy in a murine model. We demonstrated that daily dantrolene treatment was associated with mitigation of Dox-induced aberration of cytosolic Ca2+ dynamics. This mitigation of calcium dysfunction caused by Dox was associated with protection from contractile dysfunction in our experimental model. To the best of our knowledge, this is the first study to assess a mechanistically novel therapeutic approach to prevent DICM by modulating calcium cycling using dantrolene.
Dantrolene and cardiac contractile function
In this experimental model, our findings surrounding doxorubicin-induced cardiac dysfunction were consistent with those of previous studies,8 and we found that dantrolene was indeed successful as a cardioprotective agent. A study by Sufu-Shimizu and colleagues27 demonstrated a marked improvement in FS and RyR2 function following chronic dantrolene treatment in CaMKIIδc-overexpressing mice. Interestingly, CaMKIIδc overexpression in mice was associated with increased phosphorylation of RyR2 and chronic dantrolene treatment for 1 month improved RyR2 function without changing its phosphorylation status.27 Rather, dantrolene improved the association of calmodulin with RyR2, suggesting the possible zipping of unzipped RyR2.27 In another study, it has been demonstrated that CaMKII played an important role in Dox-induced impaired Ca2+ dynamics in isolated cardiomyocytes.28 In addition, improvements in cardiac function have been demonstrated following treatment with dantrolene in a canine model of long-term pacing-induced heart failure.18 In our present study, there were no changes in contractile function and calcium dynamics in the heart of mice treated with dantrolene only. This finding is consistent with that of Kobayashi and colleagues,18 who demonstrated that dantrolene is effective in stabilizing RyR2 only in the unzipped state, but not in the zipped state. In our previous study, which used azumolene, an active analog of dantrolene, we demonstrated that the phosphorylation of RyR2 is essential for azumolene function in ischemia and long-duration ventricular fibrillation.24 The cardioprotective effect of dantrolene has also been demonstrated in various acute and chronic models of DICM,29,30 but these previous studies did not assess the impact on calcium dynamics as demonstrated in this study.
Dantrolene and systolic calcium rise
In the present study, doxorubicin treatment led to prolongation of CaT rise time. Both CaT rise time and time to peak provide an assessment of the integrity of systolic calcium release function (calcium-induced calcium release, or CICR) from the sarcoplasmic reticulum (SR).26 However, measurement of rise time is not influenced by errors from signal smoothing and imaging frame rates.26 Prolongation of systolic calcium release time is a hallmark of diseased myocardium31 and has been reported in cardiomyocytes from animals treated with doxorubicin.32, 33, 34, 35 The prolongation of rise time is linked to contractile dysfunction in infarcted and failing hearts,36,37 as well as hearts with doxorubicin-induced cardiotoxicity.32,33 Dyssynchronous Ca2+ release from RyR2 channels is associated with prolongation of systolic calcium release time in remodeled myocardium,36,37 and leaky RyR2 plays a crucial role in the prolongation of calcium release time.38,39 Synchronization of RyR2 has been reported to significantly improve myocardial contractility and shorten systolic calcium rise time in experimental models.40 Doxorubicin-induced alteration in the expression and function of RyR2 may contribute to abnormal CaT rise time. Doxorubicin is known to reduce the expression of RyR2 in cardiomyocytes41 as well as increase the open probability of the channel, thereby resulting in increased calcium leak from the SR,42,43 which can increase cardiac alternans. In addition to enhanced CaMKII phosphorylation,44 the binding of Dox to RyR2 may increase the open probability of the channel and increase the calcium leak from SR.42,43
Our previous study and others demonstrated that dantrolene can improve contractile function and reduce arrhythmia in a wide variety of acute and chronic cardiomyopathy models.19, 20, 21, 22,45 A reduction of diastolic calcium leak is also known to be associated with dantrolene treatment,22 as dantrolene prevents the pathologic unzipping of remodeled RyR2.18 In the present study, dantrolene treatment was found to reduce the CaT rise time in the doxorubicin-treated mice, but not in the control group. Therefore, the stabilization of remodeled RyR2 by dantrolene in DICM may explain the shortening of the CaT rise time and subsequent improvement in myocardial contractility. The protective effect of dantrolene against doxorubicin-induced CaT remodeling is a novel finding from the present study.
Dantrolene and calcium transient duration
Our study also demonstrated the prolongation of CaTD50 and CaTD80 by doxorubicin. Prolongation of CaTD50 and CaTD80 are indicative of impairment in diastolic Ca2+ extrusion.26 Doxorubicin may prolong CaTD by inhibiting SR Ca2+ uptake or by promoting persistent Ca2+ leak from SR or by prolongation of CaT rise time. Mechanisms of abnormal Ca2+ sequestration induced by doxorubicin include inhibition of SERCA2a expression, redox modification of SERCA2a, impaired SERCA2a activity from cellular ATP depletion, and inhibition of Na+/Ca2+ exchanger.46, 47, 48, 49 The prolongation of the diastolic phase of the calcium transient is linked to diastolic dysfunction in cardiomyocytes of doxorubicin-treated rats.34 The presence of diastolic dysfunction is considered a poor prognostic marker in heart failure and has been reported to precede contractile dysfunction in DICM.50 Concurrent dantrolene administration prevented the doxorubicin-induced prolongation of CaTD in our study. A previous study demonstrated that dantrolene enhanced SR Ca2+ uptake in a rat heart.51 Dantrolene has also been found to preserve SERCA2a expression following isoprenaline treatment52 and the prevention of diastolic calcium leak by stabilization of remodeled RyR2 is expected to reduce the CaTD following doxorubicin-induced cardiomyopathy.
Dantrolene and calcium alternans
Our study also demonstrated the promotion of CaT amplitude alternans following doxorubicin treatment and its subsequent inhibition following dantrolene administration in the heart of doxorubicin-treated mice. CaT amplitude alternans is a consequence of dysfunction in the SR Ca2+ handling proteins53,54 and leads to the generation of mechanical alternans.55 In the myopathic heart, the presence of mechanical alternans indicates an advanced disease stage56 and is associated with an increased risk of adverse events.57 Mitigation of Ca2+ amplitude alternans by RyR2 stabilization may indicate a restorative effect on calcium handling after a myopathic insult following doxorubicin treatment.
Limitations
There are some limitations in this study that should be noted. Firstly, doxorubicin was administered as a single injection rather than with repeated smaller doses. Despite the brief treatment, this model exhibited persistent LV dysfunction following doxorubicin injection. Secondly, our experimental model is not a cancer model, and we cannot determine if dantrolene interferes with the chemotherapeutic effects associated with doxorubicin treatment. However, in a previous study using a breast cancer model, dantrolene was not found to influence the antitumor activity of doxorubicin.29 Lastly, given that we only included male mice in our experiment, the results of our experiment may suffer the limitation of generalizability. This is owing to differences in vulnerability and clinical course of doxorubicin-induced cardiomyopathy in males and females.58,59
Conclusion
Intracellular calcium dysregulation plays a crucial role in the pathogenesis of doxorubicin-induced cardiomyopathy. Restoration of calcium dynamics via dantrolene administration is successful in preventing cardiotoxicity following doxorubicin treatment.
Acknowledgments
K. Nanthakumar is a recipient of the Mid-Career Investigator Award from the Heart and Stroke Foundation of Ontario. We thank Arulalan Veluppillai for his assistance in the preparation of various figures in this manuscript.
Funding Sources
This work was funded by the Canadian Institutes of Health Research (CIHR, MOP 142272) and the Hang Tough initiative.
Disclosures
K.N. is a consultant for Servier, Biosense Webster, Abbott, and BlueRock. S.M. is a consultant for Abbott. S.R. has received consulting fee from Norgine Pharmaceuticals. The remaining authors have nothing to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Ethics Statement
All animal usage was in accordance with institutional animal care guidelines and were approved by the Animal Care Committee of the University Health Network (Canadian Council in Animal Care).
References
- 1.McGowan J.V., Chung R., Maulik A., et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henriksen P.A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104:971–977. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- 3.Mitry M.A., Edwards J.G. Doxorubicin induced heart failure: phenotype and molecular mechanisms. Int J Cardiol Heart Vasc. 2016;10:17–24. doi: 10.1016/j.ijcha.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai M., Tomaru K., Takizawa T., et al. Sarcoplasmic reticulum genes are selectively down-regulated in cardiomyopathy produced by doxorubicin in rabbits. J Mol Cell Cardiol. 1998;30:243–254. doi: 10.1006/jmcc.1997.0588. [DOI] [PubMed] [Google Scholar]
- 5.Olson H.M., Young D.M., Prieur D.J., LeRoy A.F., Reagan R.L. Electrolyte and morphologic alterations of myocardium in adriamycin-treated rabbits. Am J Pathol. 1974;77:439–454. [PMC free article] [PubMed] [Google Scholar]
- 6.Kalivendi S.V., Konorev E.A., Cunningham S., et al. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.Y., Kim S.J., Kim B.J., et al. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Exp Mol Med. 2006;38:535–545. doi: 10.1038/emm.2006.63. [DOI] [PubMed] [Google Scholar]
- 8.Sabatino J., De Rosa S., Tamme L., et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc Diabetol. 2020;19:66. doi: 10.1186/s12933-020-01040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dadson K., Thavendiranathan P., Azam M.A., et al. Statins mediate recovery from chemotheraphy-induced cardiotoxicity. Can J Cardiol. 2018;34:S31. [Google Scholar]
- 10.Azam M.A., Dadson K., Du B., et al. Doxorubicin-induced cardiomyopathy: calcium dynamics and ryanodine receptor dysfunction. Heart Rhythm. 2019;16:S339. [Google Scholar]
- 11.Luo M., Anderson M.E. Mechanisms of altered Ca2+ handling in heart failure. Circ Res. 2013;113:690–708. doi: 10.1161/CIRCRESAHA.113.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Octavia Y., Tocchetti C.G., Gabrielson K.L., et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Derek M., Yellon J., Sapna A. Preventing the cancer patient of today from becoming the heart failure patient of tomorrow. JACC Cardiol. 2019;1:235–237. [Google Scholar]
- 14.Avila M.S., Ayub-Ferreira S.M., de Barros Wanderley M.R., Jr., et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71:2281–2290. doi: 10.1016/j.jacc.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 15.van Dalen E.C., Caron H.N., Dickinson H.O., Kremer L.C. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011:CD003917. doi: 10.1002/14651858.CD003917.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Bjelogrlic S.K., Radic J., Jovic V., Radulovic S. Activity of d,l-alpha-tocopherol (vitamin E) against cardiotoxicity induced by doxorubicin and doxorubicin with cyclophosphamide in mice. Basic Clin Pharmacol Toxicol. 2005;97:311–319. doi: 10.1111/j.1742-7843.2005.pto_166.x. [DOI] [PubMed] [Google Scholar]
- 17.Goey A.K., Schellens J.H., Beijnen J.H., Huitema A.D. [Dexrazoxane in anthracycline induced cardiotoxicity and extravasation] Ned Tijdschr Geneeskd. 2010;154:A1155. [PubMed] [Google Scholar]
- 18.Kobayashi S., Yano M., Suetomi T., et al. Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol. 2009;53:1993–2005. doi: 10.1016/j.jacc.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domeier T.L., Roberts C.J., Gibson A.K., et al. Dantrolene suppresses spontaneous Ca2+ release without altering excitation-contraction coupling in cardiomyocytes of aged mice. Am J Physiol Heart Circ Physiol. 2014;307:H818–H829. doi: 10.1152/ajpheart.00287.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meissner A., Min J.Y., Haake N., Hirt S., Simon R. Dantrolene sodium improves the force-frequency relationship and beta-adregenic responsiveness in failing human myocardium. Eur J Heart Fail. 1999;1:177–186. doi: 10.1016/s1388-9842(99)00017-3. [DOI] [PubMed] [Google Scholar]
- 21.Nofi C., Zhang K., Tang Y., et al. Chronic dantrolene treatment attenuates cardiac dysfunction and reduces atrial fibrillation inducibility in a rat myocardial infarction heart failure model. Heart Rhythm O2. 2020:126–135. doi: 10.1016/j.hroo.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamiri N., Masse S., Ramadeen A., et al. Dantrolene improves survival after ventricular fibrillation by mitigating impaired calcium handling in animal models. Circulation. 2014;129:875–885. doi: 10.1161/CIRCULATIONAHA.113.005443. [DOI] [PubMed] [Google Scholar]
- 23.Azam M.A., Zamiri N., Masse S., et al. Effects of late sodium current blockade on ventricular refibrillation in a rabbit model. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004331. [DOI] [PubMed] [Google Scholar]
- 24.Si D., Azam M.A., Lai P.F.H., et al. Essential role of ryanodine receptor 2 phosphorylation in the effect of azumolene on ventricular arrhythmia vulnerability in a rabbit heart model. J Cardiovasc Electrophysiol. 2018;29:1707–1715. doi: 10.1111/jce.13737. [DOI] [PubMed] [Google Scholar]
- 25.Azam M.A., Chakraborty P., Si D., et al. Anti-arrhythmic and inotropic effects of empagliflozin following myocardial ischemia. Life Sci. 2021;276:119440. doi: 10.1016/j.lfs.2021.119440. [DOI] [PubMed] [Google Scholar]
- 26.Jaimes R., 3rd, Walton R.D., Pasdois P., et al. A technical review of optical mapping of intracellular calcium within myocardial tissue. Am J Physiol Heart Circ Physiol. 2016;310:H1388–H1401. doi: 10.1152/ajpheart.00665.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sufu-Shimizu Y., Okuda S., Kato T., et al. Stabilizing cardiac ryanodine receptor prevents the development of cardiac dysfunction and lethal arrhythmia in Ca2+/calmodulin-dependent protein kinase IIδc transgenic mice. Biochem Biophys Res Commun. 2020;524:431–438. doi: 10.1016/j.bbrc.2020.01.107. [DOI] [PubMed] [Google Scholar]
- 28.Sag C.M., Kohler A.C., Anderson M.E., Backs J., Maier L.S. CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J Mol Cell Cardiol. 2011;51:749–759. doi: 10.1016/j.yjmcc.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todorova V.K., Siegel E.R., Kaufmann Y., et al. Dantrolene attenuates cardiotoxicity of doxorubicin without reducing its antitumor efficacy in a breast cancer model. Transl Oncol. 2020;13:471–480. doi: 10.1016/j.tranon.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buyukokuroglu M.E., Taysi S., Buyukavci M., Bakan E. Prevention of acute adriamycin cardiotoxicity by dantrolene in rats. Hum Exp Toxicol. 2004;23:251–256. doi: 10.1191/0960327104ht443oa. [DOI] [PubMed] [Google Scholar]
- 31.Lou Q., Janardhan A., Efimov I.R. Remodeling of calcium handling in human heart failure. Adv Exp Med Biol. 2012;740:1145–1174. doi: 10.1007/978-94-007-2888-2_52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J., Temma K., Akera T. Doxorubicin-induced changes in intracellular Ca2+ transients observed in cardiac myocytes isolated from guinea-pig heart. Can J Physiol Pharmacol. 1994;72:622–631. doi: 10.1139/y94-088. [DOI] [PubMed] [Google Scholar]
- 33.Temma K., Chugun A., Akera T., Kondo H., Kurebayashi N. Doxorubicin alters Ca2+ transients but fails to change Ca2+ sensitivity of contractile proteins. Environ Toxicol Pharmacol. 1996;1:131–139. doi: 10.1016/1382-6689(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 34.Maeda A., Honda M., Kuramochi T., Takabatake T. Doxorubicin cardiotoxicity: diastolic cardiac myocyte dysfunction as a result of impaired calcium handling in isolated cardiac myocytes. Jpn Circ J. 1998;62:505–511. doi: 10.1253/jcj.62.505. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y.X., Korth M. Effects of doxorubicin on excitation-contraction coupling in guinea pig ventricular myocardium. Circ Res. 1995;76:645–653. doi: 10.1161/01.res.76.4.645. [DOI] [PubMed] [Google Scholar]
- 36.Mork H.K., Sjaastad I., Sejersted O.M., Louch W.E. Slowing of cardiomyocyte Ca2+ release and contraction during heart failure progression in postinfarction mice. Am J Physiol Heart Circ Physiol. 2009;296:H1069–H1079. doi: 10.1152/ajpheart.01009.2008. [DOI] [PubMed] [Google Scholar]
- 37.Louch W.E., Mork H.K., Sexton J., et al. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marx S.O., Reiken S., Hisamatsu Y., et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 39.Marx S.O., Gaburjakova J., Gaburjakova M., et al. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- 40.Jaimes R., 3rd, Kuzmiak-Glancy S., Brooks D.M., et al. Functional response of the isolated, perfused normoxic heart to pyruvate dehydrogenase activation by dichloroacetate and pyruvate. Pflugers Arch. 2016;468:131–142. doi: 10.1007/s00424-015-1717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson R.D., Gambliel H.A., Vestal R.E., et al. Doxorubicin cardiac dysfunction: effects on calcium regulatory proteins, sarcoplasmic reticulum, and triiodothyronine. Cardiovasc Toxicol. 2005;5:269–283. doi: 10.1385/ct:5:3:269. [DOI] [PubMed] [Google Scholar]
- 42.Holmberg S.R., Williams A.J. Patterns of interaction between anthraquinone drugs and the calcium-release channel from cardiac sarcoplasmic reticulum. Circ Res. 1990;67:272–283. doi: 10.1161/01.res.67.2.272. [DOI] [PubMed] [Google Scholar]
- 43.Saeki K., Obi I., Ogiku N., et al. Doxorubicin directly binds to the cardiac-type ryanodine receptor. Life Sci. 2002;70:2377–2389. doi: 10.1016/s0024-3205(02)01524-2. [DOI] [PubMed] [Google Scholar]
- 44.Gao J., Chen T., Zhao D., Zheng J., Liu Z. Ginkgolide B exerts cardioprotective properties against doxorubicin-induced cardiotoxicity by regulating reactive oxygen species, Akt and calcium signaling pathways in vitro and in vivo. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartmann N., Pabel S., Herting J., et al. Antiarrhythmic effects of dantrolene in human diseased cardiomyocytes. Heart Rhythm. 2017;14:412–419. doi: 10.1016/j.hrthm.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Arai M., Yoguchi A., Takizawa T., et al. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca2+-ATPase gene transcription. Circ Res. 2000;86:8–14. doi: 10.1161/01.res.86.1.8. [DOI] [PubMed] [Google Scholar]
- 47.Bertero E., Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. 2018;122:1460–1478. doi: 10.1161/CIRCRESAHA.118.310082. [DOI] [PubMed] [Google Scholar]
- 48.Singal P.K. Adriamycin does have a potentially depressant effect on left ventricular contractility. Int J Cardiol. 1985;7:447–449. doi: 10.1016/0167-5273(85)90103-2. [DOI] [PubMed] [Google Scholar]
- 49.Caroni P., Villani F., Carafoli E. The cardiotoxic antibiotic doxorubicin inhibits the Na+/Ca2+ exchange of dog heart sarcolemmal vesicles. FEBS Lett. 1981;130:184–186. doi: 10.1016/0014-5793(81)81115-5. [DOI] [PubMed] [Google Scholar]
- 50.Lee B.H., Goodenday L.S., Muswick G.J., et al. Alterations in left ventricular diastolic function with doxorubicin therapy. J Am Coll Cardiol. 1987;9:184–188. doi: 10.1016/s0735-1097(87)80099-2. [DOI] [PubMed] [Google Scholar]
- 51.Meissner A., Szymanska G., Morgan J.P. Effects of dantrolene sodium on intracellular Ca2+-handling in normal and Ca2+-overloaded cardiac muscle. Eur J Pharmacol. 1996;316:333–342. doi: 10.1016/s0014-2999(96)00678-4. [DOI] [PubMed] [Google Scholar]
- 52.Liu T., Shi S.B., Qin M., Huang C.X. Effects of dantrolene treatment on ventricular electrophysiology and arrhythmogenesis in rats with chronic beta-adrenergic receptor activation. J Cardiovasc Pharmacol Ther. 2015;20:414–427. doi: 10.1177/1074248414568194. [DOI] [PubMed] [Google Scholar]
- 53.Diaz M.E., O'Neill S.C., Eisner D.A. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 54.Sun B., Wei J., Zhong X., et al. The cardiac ryanodine receptor, but not sarcoplasmic reticulum Ca2+-ATPase, is a major determinant of Ca2+ alternans in intact mouse hearts. J Biol Chem. 2018;293:13650–13661. doi: 10.1074/jbc.RA118.003760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kihara Y., Morgan J.P. Abnormal Ca2+i handling is the primary cause of mechanical alternans: study in ferret ventricular muscles. Am J Physiol. 1991;261:H1746–H1755. doi: 10.1152/ajpheart.1991.261.6.H1746. [DOI] [PubMed] [Google Scholar]
- 56.Kodama M., Kato K., Hirono S., et al. Mechanical alternans in patients with chronic heart failure. J Card Fail. 2001;7:138–145. doi: 10.1054/jcaf.2001.24122. [DOI] [PubMed] [Google Scholar]
- 57.Kim R., Cingolani O., Wittstein I., et al. Mechanical alternans is associated with mortality in acute hospitalized heart failure: prospective mechanical alternans study (MAS) Circ Arrhythm Electrophysiol. 2014;7:259–266. doi: 10.1161/CIRCEP.113.000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipshultz S.E., Lipsitz S.R., Mone S.M., et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 59.Silber J.H., Jakacki R.I., Larsen R.L., Goldwein J.W., Barber G. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol. 1993;21:477–479. doi: 10.1002/mpo.2950210704. [DOI] [PubMed] [Google Scholar]