Extended Data Figure 6 |. Affinity of orthosteric ligands at mutations of the MIPS521 extrahelical allosteric binding pocket.
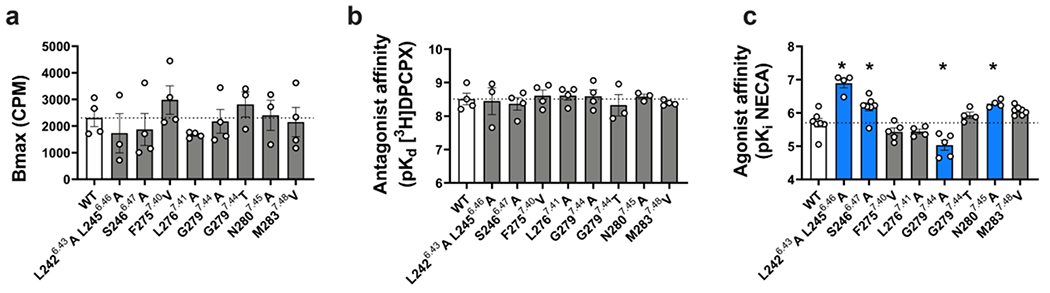
a, c, The affinity of (a) [3H]DPCPX and (c) NECA for wildtype and mutant A1Rs performed in FlpInCHO cells. b, Bmax; determined by [3H]DPCPX radioligand saturation binding studies. Data are the means + S.E.M. of 3-7 independent experiments (shown as circles) performed in duplicate. *P < 0.05 (compared with WT; one-way analysis of variance, Dunnett’s post hoc test).
