Figure 2 |. Comparison of the structures of the A1R-Gi2 complex in the presence and absence of the positive allosteric modulator, MIPS521.
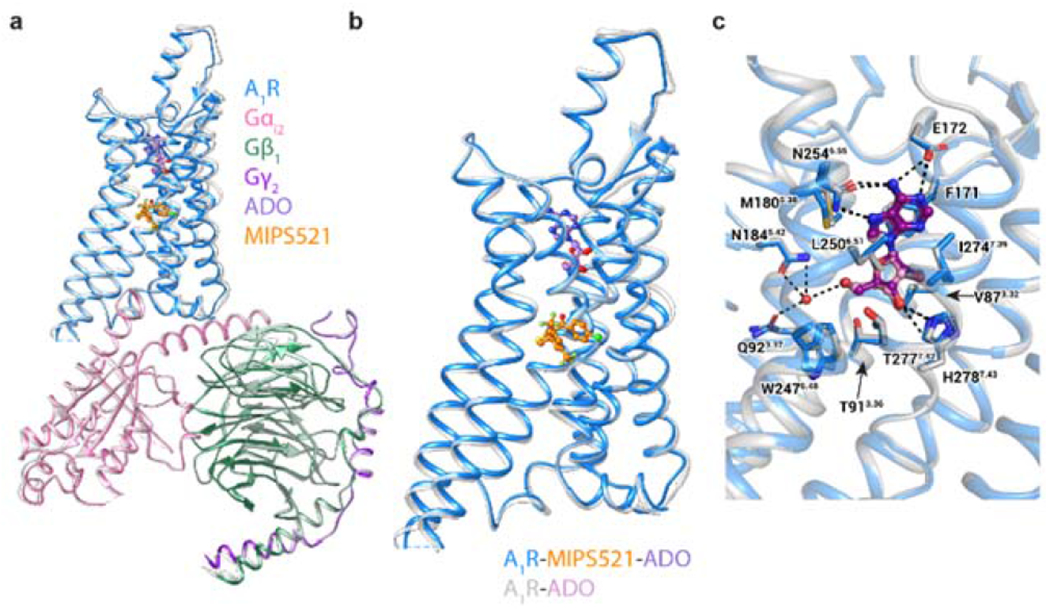
a, b, Overlay of the A1R-Gi2 complex in the presence and absence of MIPS521, showing (a) the whole complex and (b) receptor alone (in MIPS521-ADO-A1R-Gi2 the receptor is blue and the heterotrimeric Gi2 pink, cyan and dark purple for α, β and γ, respectively, ADO in purple and MIPS521 in orange; the ADO-A1R-Gi2 complex is coloured grey, with ADO in plum), c, The orthosteric binding site of the A1R-Gi2 complex in the presence and absence of MIPS521 is highly conserved. Water molecule shown as red sphere. ADO is shown in ball and stick representation and residues of the orthosteric binding pocket in stick representation, coloured by heteroatom.
